-
 Rose stretches lead at Torrey Pines as Koepka makes cut
Rose stretches lead at Torrey Pines as Koepka makes cut
-
Online foes Trump, Petro set for White House face-to-face

-
 Seattle Seahawks deny plans for post-Super Bowl sale
Seattle Seahawks deny plans for post-Super Bowl sale
-
US Senate passes deal expected to shorten shutdown

-
 'Misrepresent reality': AI-altered shooting image surfaces in US Senate
'Misrepresent reality': AI-altered shooting image surfaces in US Senate
-
Thousands rally in Minneapolis as immigration anger boils

-
 US judge blocks death penalty for alleged health CEO killer Mangione
US judge blocks death penalty for alleged health CEO killer Mangione
-
Lens win to reclaim top spot in Ligue 1 from PSG

-
 Gold, silver prices tumble as investors soothed by Trump Fed pick
Gold, silver prices tumble as investors soothed by Trump Fed pick
-
Ko, Woad share lead at LPGA season opener

-
 US Senate votes on funding deal - but shutdown still imminent
US Senate votes on funding deal - but shutdown still imminent
-
US charges prominent journalist after Minneapolis protest coverage

-
 Trump expects Iran to seek deal to avoid US strikes
Trump expects Iran to seek deal to avoid US strikes
-
US Justice Dept releases documents, images, videos from Epstein files

-
 Guterres warns UN risks 'imminent financial collapse'
Guterres warns UN risks 'imminent financial collapse'
-
NASA delays Moon mission over frigid weather

-
 First competitors settle into Milan's Olympic village
First competitors settle into Milan's Olympic village
-
Fela Kuti: first African to get Grammys Lifetime Achievement Award

-
 Cubans queue for fuel as Trump issues oil ultimatum
Cubans queue for fuel as Trump issues oil ultimatum
-
'Schitt's Creek' star Catherine O'Hara dead at 71

-
 Curran hat-trick seals 11 run DLS win for England over Sri Lanka
Curran hat-trick seals 11 run DLS win for England over Sri Lanka
-
Cubans queue for fuel as Trump issues energy ultimatum

-
 France rescues over 6,000 UK-bound Channel migrants in 2025
France rescues over 6,000 UK-bound Channel migrants in 2025
-
Surprise appointment Riera named Frankfurt coach

-
 Maersk to take over Panama Canal port operations from HK firm
Maersk to take over Panama Canal port operations from HK firm
-
US arrests prominent journalist after Minneapolis protest coverage

-
 Analysts say Kevin Warsh a safe choice for US Fed chair
Analysts say Kevin Warsh a safe choice for US Fed chair
-
Trump predicts Iran will seek deal to avoid US strikes

-
 US oil giants say it's early days on potential Venezuela boom
US oil giants say it's early days on potential Venezuela boom
-
Fela Kuti to be first African to get Grammys Lifetime Achievement Award

-
 Trump says Iran wants deal, US 'armada' larger than in Venezuela raid
Trump says Iran wants deal, US 'armada' larger than in Venezuela raid
-
US Justice Dept releases new batch of documents, images, videos from Epstein files

-
 Four memorable showdowns between Alcaraz and Djokovic
Four memorable showdowns between Alcaraz and Djokovic
-
Russian figure skating prodigy Valieva set for comeback -- but not at Olympics

-
 Barcelona midfielder Lopez agrees contract extension
Barcelona midfielder Lopez agrees contract extension
-
Djokovic says 'keep writing me off' after beating Sinner in late-nighter

-
 US Justice Dept releasing new batch of Epstein files
US Justice Dept releasing new batch of Epstein files
-
South Africa and Israel expel envoys in deepening feud

-
 French eyewear maker in spotlight after presidential showing
French eyewear maker in spotlight after presidential showing
-
Olympic dream 'not over', Vonn says after crash

-
 Brazil's Lula discharged after cataract surgery
Brazil's Lula discharged after cataract surgery
-
US Senate races to limit shutdown fallout as Trump-backed deal stalls

-
 'He probably would've survived': Iran targeting hospitals in crackdown
'He probably would've survived': Iran targeting hospitals in crackdown
-
Djokovic stuns Sinner to set up Australian Open final with Alcaraz

-
 Mateta omitted from Palace squad to face Forest
Mateta omitted from Palace squad to face Forest
-
Gold, silver prices tumble as investors soothed by Trump's Fed pick

-
 Trump attorney general orders arrest of ex-CNN anchor covering protests
Trump attorney general orders arrest of ex-CNN anchor covering protests
-
Djokovic 'pushed to the limit' in stunning late-night Sinner upset

-
 Tunisia's famed blue-and-white village threatened after record rains
Tunisia's famed blue-and-white village threatened after record rains
-
Top EU official voices 'shock' at Minneapolis violence


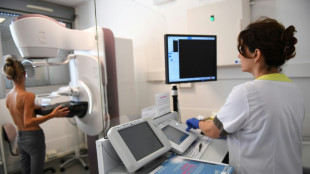
US limits Covid boosters to over-65s or those at high risk
The United States will limit Covid-19 boosters to people over 65 or those at risk of serious illness, while requiring vaccine makers to run fresh clinical trials before offering shots to younger and healthier individuals, officials said Tuesday.
Writing in the New England Journal of Medicine, the Food and Drug Administration's Vinayak Prasad and Commissioner Martin Makary framed the policy shift as "evidence-based" and would align the United States more closely with guidance in Europe.
But it comes as Health Secretary Robert F. Kennedy Jr., a longtime vaccine skeptic, pushes to remake federal public health policy.
Kennedy previously led a nonprofit that was critical of immunization programs, and during the pandemic petitioned the FDA to revoke Covid vaccine authorizations, citing rare side effects including heart inflammation.
Prasad and Makary praised the initial Covid-19 vaccine rollout as "a major scientific, medical, and regulatory accomplishment," but argued that the benefits of repeated boosters for low-risk individuals are uncertain.
They criticized the US approach of recommending boosters for all adults regardless of age or health status, calling it a "one-size-fits-all" model based on the mistaken belief that Americans couldn't handle more nuanced, risk-based advice.
Rather than building public trust, they wrote, it had backfired -- fueling vaccine hesitancy that has spilled over into skepticism toward childhood shots, including those for measles.
The FDA said it would rely on lab test results to approve boosters for people who are over 65, or over six months old with at least one underlying condition.
But for healthy individuals between six months and 64 years, regulators will now require data from randomized trials.
"We simply don't know whether a healthy 52-year-old woman with a normal BMI (body mass index) who has had Covid-19 three times and has received six previous doses of a Covid-19 vaccine will benefit from the seventh dose," they wrote.
Some infectious disease experts welcomed the shift.
Amesh Adalja of Johns Hopkins University said it matched with the approach taken by other countries in a population that already carries significant immunity.
"For lower-risk individuals, the goal has always been less clear, as protection against infection is transient and they don't have a high risk of severe disease," he told AFP.
But others voiced concern about the practical consequences. Paul Offit, a leading vaccine expert at the Children's Hospital of Philadelphia, said it could limit access for people who still want boosters.
"Any use, say in a healthy 35-year-old, would be considered off-label, and you wonder whether an insurance company would pay for it," he told AFP.
- Not like annual flu shot -
Under the revised framework, companies like Pfizer and Moderna will be encouraged to test updated boosters in adults aged 50 to 64.
These studies should measure whether the vaccines reduce symptomatic infections, hospitalizations and deaths.
Rather than comparing new shots to earlier formulations, Prasad and Makary suggested placebo-controlled trials -- with saline as the comparator -- to better evaluate both benefit and potential side effects.
The proposal, first floated by Kennedy earlier this month, has proved divisive. Critics argue that using a placebo -- when authorized vaccines already exist -- could expose participants to unnecessary harm.
"Imagine if there was a death or two in the placebo group," said Offit. "I don't see how you conscience that."
Supporters of continued Covid-19 boosters often draw parallels to annual flu shots.
But Makary and Prasad pushed back on that comparison, arguing the genetic changes in Covid variants haven’t been significant enough to justify automatically updating the vaccine each year.
The FDA officials also sought to reassure Americans concerned they might lose access to boosters under the new framework.
The Centers for Disease Control and Prevention's (CDC) definition of risk factors is "vast, including obesity and even mental health conditions such as depression," they wrote, noting that between 100 million and 200 million Americans would likely still qualify.
N.Mitchell--AT