
-
 McLaren will make 'practical' call on team orders in Abu Dhabi, says boss Brown
McLaren will make 'practical' call on team orders in Abu Dhabi, says boss Brown
-
Norris completes Abu Dhabi practice 'double top' to boost title bid

-
 Chiba leads Liu at skating's Grand Prix Final
Chiba leads Liu at skating's Grand Prix Final
-
Meta partners with news outlets to expand AI content

-
 Mainoo 'being ruined' at Man Utd: Scholes
Mainoo 'being ruined' at Man Utd: Scholes
-
Guardiola says broadcasters owe him wine after nine-goal thriller

-
 Netflix to buy Warner Bros. Discovery in deal of the decade
Netflix to buy Warner Bros. Discovery in deal of the decade
-
French stars Moefana and Atonio return for Champions Cup

-
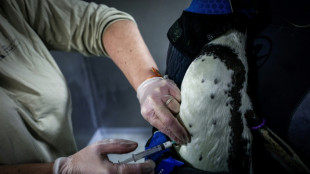 Penguins queue in Paris zoo for their bird flu jabs
Penguins queue in Paris zoo for their bird flu jabs
-
Netflix to buy Warner Bros. Discovery for nearly $83 billion

-
 Sri Lanka issues fresh landslide warnings as toll nears 500
Sri Lanka issues fresh landslide warnings as toll nears 500
-
Root says England still 'well and truly' in second Ashes Test

-
 Chelsea's Maresca says rotation unavoidable
Chelsea's Maresca says rotation unavoidable
-
Italian president urges Olympic truce at Milan-Cortina torch ceremony

-
 Norris edges Verstappen in opening practice for season-ending Abu Dhabi GP
Norris edges Verstappen in opening practice for season-ending Abu Dhabi GP
-
Australia race clear of England to seize control of second Ashes Test

-
 Stocks, dollar rise before key US inflation data
Stocks, dollar rise before key US inflation data
-
Trump strategy shifts from global role and vows 'resistance' in Europe

-
 Turkey orders arrest of 29 footballers in betting scandal
Turkey orders arrest of 29 footballers in betting scandal
-
EU hits X with 120-mn-euro fine, risking Trump ire

-
 Arsenal's Merino has earned striking role: Arteta
Arsenal's Merino has earned striking role: Arteta
-
Putin offers India 'uninterrupted' oil in summit talks with Modi

-
 New Trump strategy vows shift from global role to regional
New Trump strategy vows shift from global role to regional
-
World Athletics ditches long jump take-off zone reform

-
 French town offers 1,000-euro birth bonuses to save local clinic
French town offers 1,000-euro birth bonuses to save local clinic
-
After wins abroad, Syria leader must gain trust at home

-
 Slot spots 'positive' signs at struggling Liverpool
Slot spots 'positive' signs at struggling Liverpool
-
Eyes of football world on 2026 World Cup draw with Trump centre stage

-
 South Africa rugby coach Erasmus extends contract until 2031
South Africa rugby coach Erasmus extends contract until 2031
-
Ex-Manchester Utd star Lingard announces South Korea exit

-
 Australia edge ominously within 106 runs of England in second Ashes Test
Australia edge ominously within 106 runs of England in second Ashes Test
-
Markets rise ahead of US data, expected Fed rate cut
-
 McIlroy survives as Min Woo Lee surges into Australian Open hunt
McIlroy survives as Min Woo Lee surges into Australian Open hunt
-
German factory orders rise more than expected

-
 India's Modi and Russia's Putin talk defence, trade and Ukraine
India's Modi and Russia's Putin talk defence, trade and Ukraine
-
Flooding kills two as Vietnam hit by dozens of landslides

-
 Italy to open Europe's first marine sanctuary for dolphins
Italy to open Europe's first marine sanctuary for dolphins
-
Hong Kong university suspends student union after calls for fire justice

-
 Asian markets rise ahead of US data, expected Fed rate cut
Asian markets rise ahead of US data, expected Fed rate cut
-
Nigerian nightlife finds a new extravagance: cabaret

-
 Tanzania tourism suffers after election killings
Tanzania tourism suffers after election killings
-
Yo-de-lay-UNESCO? Swiss hope for yodel heritage listing

-
 Weatherald fires up as Australia race to 130-1 in second Ashes Test
Weatherald fires up as Australia race to 130-1 in second Ashes Test
-
Georgia's street dogs stir affection, fear, national debate

-
 Survivors pick up pieces in flood-hit Indonesia as more rain predicted
Survivors pick up pieces in flood-hit Indonesia as more rain predicted
-
Gibbs runs for three TDs as Lions down Cowboys to boost NFL playoff bid

-
 Pandas and ping-pong: Macron ending China visit on lighter note
Pandas and ping-pong: Macron ending China visit on lighter note
-
TikTok to comply with 'upsetting' Australian under-16 ban

-
 Hope's resistance keeps West Indies alive in New Zealand Test
Hope's resistance keeps West Indies alive in New Zealand Test
-
Pentagon endorses Australia submarine pact


C. Light Named One of Five Selected Projects in the Roche MS Innovation Challenge
Two-Year Clinical Trial with UCSF and Sutter Health Will Study Fixational Eye Movements as a Biomarker of MS Progression
SANTA CLARA, CA / ACCESS Newswire / November 19, 2025 / C. Light Technologies, an AI-driven healthtech company specializing in assessing eye and brain health to aid in early detection and earlier intervention of neurodegenerative diseases, has been selected as one of five project teams that will be funded through the Roche MS Innovation Challenge. C. Light will use the funding to launch a two-year, multi-site clinical trial, partnering with leading multiple sclerosis (MS) centers at UCSF and Sutter Health, Palo Alto, Calif. to identify hard-to-detect, fixational eye movement outputs as non-invasive biomarkers for MS progression.
The Roche MS Innovation Challenge is a global initiative designed to accelerate breakthroughs that improve the lives of people with MS. It aims to bring forward practical innovations that could be implemented in clinical care within the next few years. The research trial will use C. Light's proprietary Retitrack™ scanning laser ophthalmoscopy (SLO) technology-the first FDA 510k-cleared retinal eye-movement monitor-to record eye movements at five timepoints over the course of two years.
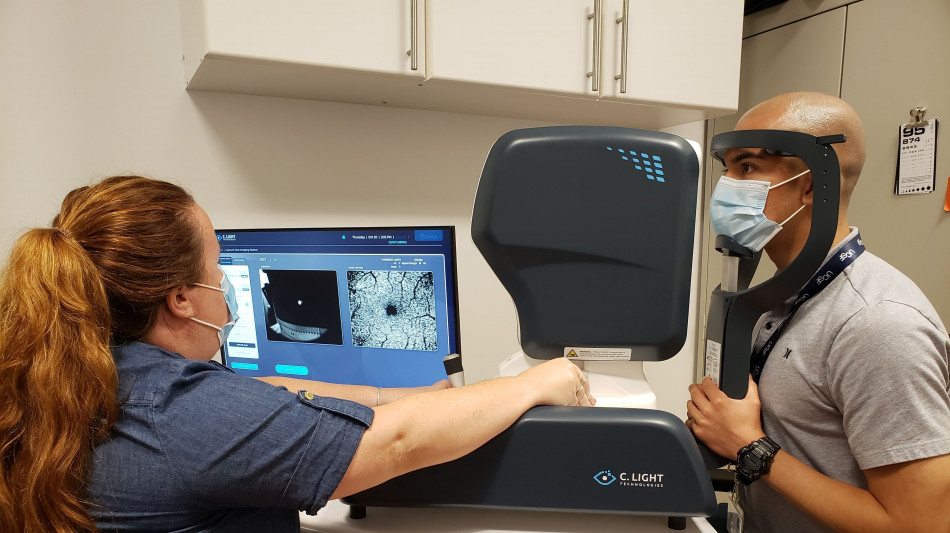
The Retitrack scanning laser ophthalmoscopy (SLO) technology
The study will aim to correlate changes in microscopic eye movements with subtle signs of PIRA (progression independent of relapse activity), including individuals already treating their condition with B-cell depleting therapies. It aims to determine if Retitrack™-detected fixational and saccadic eye-motion changes will predict PIRA earlier than the Expanded Disability Status Scale (EDSS)-the widely accepted standardized measure providing a quantitative evaluation of MS disability level-and MRI.
C. Light has shown in earlier research studies that fixational eye movements change with MS and correlate with EDSS progression. The Retitrack™ captures one of the smallest motor movements the human body can make-fixation; tiny, involuntary eye movements that can be smaller than half the size of a human hair. It measures this movement with a 10-second, non-invasive eye scan, requiring no eye dilation.
"We are creating an entirely new stream of data for healthcare," said C. Light Founder and CEO Christy Sheehy-Bensinger. "Why wait until ambulation and mobility are already impacted in a patient to document disease progression and disability? Let's use the subclinical changes of oculomotor function and fixation to detect subtle MS progression as soon as it occurs."
In July, the American Medical Association (AMA) clinical board granted C. Light a new CPT code (1010T) for retinal eye tracking, which will become effective for clinical use on Jan. 1, 2026. This opens the door for potential insurance reimbursement for Retitrack scans.
C. Light's Chief Medical Officer Jacqueline Theis, O.D., F.A.A.O. said, "Retitrack's ability to quantify microscopic fixational eye-movement changes offers a new lens on PIRA and MS detection -one that could shorten trial endpoints, refine therapeutic evaluation, and enable more personalized patient monitoring. This is the kind of bench-to-bedside innovation that has the power to transform both the science of drug development and the lived experience of MS."
In May, C. Light closed an oversubscribed $3 million seed extension round led by a large North American family office to fuel their upcoming FDA clinical trial to add AI-powered disease detection capabilities to their device.
C. Light is also announcing its participation in the DayOne Accelerator in Basel, Switzerland, a three-month, hybrid program that prepares startups to launch successful partnerships with pharmaceutical companies. Dr. Sheehy will be attending their Innovation Showcase Dec. 10 and 11 at the Roche campus in Basel, Switzerland.
About C. Light
C. Light Technologies, Inc. is an AI-driven healthtech company whose primary mission is to create digital tools and solutions to assess eye and brain health. Its proprietary Retitrack™ device is reshaping the landscape of eye-tracking, enabling clinicians to unlock the untapped potential within each eye movement. C. Light has raised more than $8 million in funding with major investors Yamaha Motor Ventures and Creative Ventures. The company has been awarded three grants from the National Institute of Health, as well as additional funding through the Alzheimer's Drug Discovery Foundation. C. Light has also won numerous awards in recent years, including the 2022 National Timmy Award for "Best Tech Startup in North America" and the 2023 Stevie® Award for Most Innovative Company of the Year - 10 or Less Employees.
For more information, visit our website at www.clighttechnologies.com or our LinkedIn.
Disclaimer: The information described above is conducted as research through controlled studies and is not intended to reflect the ability of the current commercially available iteration of the Retitrack.
Images: LINK
CONTACT:
Erica Zeidenberg
+19255188159
[email protected]
SOURCE: C. Light Technologies
View the original press release on ACCESS Newswire
W.Nelson--AT



