
-
 Australia bans under-16s from social media in world-first crackdown
Australia bans under-16s from social media in world-first crackdown
-
US Fed appears set for third rate cut despite sharp divides

-
 Veggie 'burgers' at stake in EU negotiations
Veggie 'burgers' at stake in EU negotiations
-
Haitians dance with joy over UNESCO musical listing
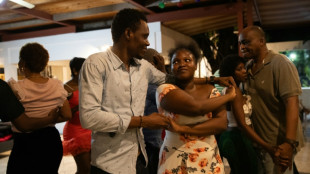
-
 Suspense swirls if Nobel peace laureate will attend ceremony
Suspense swirls if Nobel peace laureate will attend ceremony
-
UK public urged to keep eyes peeled for washed-up bananas

-
 South Korea chip giant SK hynix mulls US stock market listing
South Korea chip giant SK hynix mulls US stock market listing
-
Captain Cummins back in Australia squad for third Ashes Test

-
 NFL Colts to bring 44-year-old QB Rivers out of retirement: reports
NFL Colts to bring 44-year-old QB Rivers out of retirement: reports
-
West Indies 92-2 after being asked to bat in second New Zealand Test

-
 Ruckus in Brazil Congress over bid to reduce Bolsonaro jail term
Ruckus in Brazil Congress over bid to reduce Bolsonaro jail term
-
ExxonMobil slows low-carbon investment push through 2030

-
 Liverpool's Slot swerves further Salah talk after late Inter win
Liverpool's Slot swerves further Salah talk after late Inter win
-
Maresca concerned as Atalanta fight back to beat Chelsea

-
 Liverpool edge Inter in Champions League as Chelsea lose in Italy
Liverpool edge Inter in Champions League as Chelsea lose in Italy
-
Spurs sink Slavia Prague to boost last-16 bid in front of Son

-
 Arsenal ensure Women's Champions League play-off berth
Arsenal ensure Women's Champions League play-off berth
-
Late penalty drama helps Liverpool defy Salah crisis at angry Inter

-
 Canada launches billion dollar plan to recruit top researchers
Canada launches billion dollar plan to recruit top researchers
-
Liverpool defy Salah crisis by beating Inter Milan in Champions League

-
 Honduran leader alleges vote tampering, US interference
Honduran leader alleges vote tampering, US interference
-
De Ketelaere inspires Atalanta fightback to beat Chelsea

-
 Kounde double helps Barcelona claim Frankfurt comeback win
Kounde double helps Barcelona claim Frankfurt comeback win
-
US Supreme Court weighs campaign finance case

-
 Zelensky says ready to hold Ukraine elections, with US help
Zelensky says ready to hold Ukraine elections, with US help
-
Autistic Scottish artist Nnena Kalu smashes Turner Prize 'glass ceiling'

-
 Trump slams 'decaying' and 'weak' Europe
Trump slams 'decaying' and 'weak' Europe
-
Injury-hit Arsenal in 'dangerous circle' but Arteta defends training methods

-
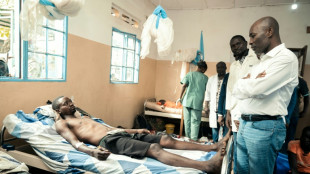 Thousands flee DR Congo fighting as M23 enters key city
Thousands flee DR Congo fighting as M23 enters key city
-
Karl and Gnabry spark Bayern to comeback win over Sporting

-
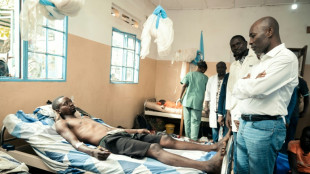 Thousands flee DR Congo fighting as M23 closes on key city
Thousands flee DR Congo fighting as M23 closes on key city
-
Zelensky says ready to hold Ukraine elections

-
 Indigenous artifacts returned by Vatican unveiled in Canada
Indigenous artifacts returned by Vatican unveiled in Canada
-
Ivory Coast recall Zaha for AFCON title defence

-
 Communist vs Catholic - Chile prepares to choose a new president
Communist vs Catholic - Chile prepares to choose a new president
-
Trump's FIFA peace prize breached neutrality, claims rights group

-
 NHL 'optimistic' about Olympic rink but could pull out
NHL 'optimistic' about Olympic rink but could pull out
-
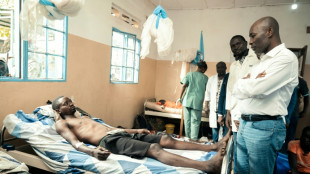
Thousands reported to have fled DR Congo fighting as M23 closes on key city
-
 Three face German court on Russia spying charges
Three face German court on Russia spying charges
-
Amy Winehouse's father sues star's friends for auctioning her clothes

-
 Woltemade's 'British humour' helped him fit in at Newcastle - Howe
Woltemade's 'British humour' helped him fit in at Newcastle - Howe
-
UK trial opens in dispute over Jimi Hendrix recordings

-
 Pandya blitz helps India thrash South Africa in T20 opener
Pandya blitz helps India thrash South Africa in T20 opener
-
Zelensky says will send US revised plan to end Ukraine war

-
 Nobel event cancellation raises questions over Machado's whereabouts
Nobel event cancellation raises questions over Machado's whereabouts
-
Miami's Messi wins second consecutive MLS MVP award

-
 Trump slams 'decaying' Europe and pushes Ukraine on elections
Trump slams 'decaying' Europe and pushes Ukraine on elections
-
TotalEnergies in deal for Namibia offshore oil field

-
 Jesus added to Arsenal's Champions League squad
Jesus added to Arsenal's Champions League squad
-
Red Bull part ways with influential advisor Marko


Revalia Bio Raises $14.5M Seed Round to Launch Human Data Trial Platform and Redefine Drug Development
NEW HAVEN, CT / ACCESS Newswire / September 4, 2025 / Revalia Bio Inc., announced a 14.5M seed funding round to support their launch of 'Human Data Trials' - a new category of pre-clinical research that gives drug developers early, predictive insights from real, functional human organs. The round was co-led by America's Frontier Fund and Sierra Ventures with participation from Roger Ferguson, former Vice Chair of the U.S. Federal Reserve and a member of the Board of Directors at Alphabet, and other existing investors. This brings Revalia's total funding to $19.5 million.
Despite billions spent on R&D each year, more than 90 percent of drug candidates fail to reach market approval. One of the key reasons is the poor translation from preclinical models to human biology. Animal models and in vitro systems often fall short in predicting how therapies will behave in real patients, leading to delays, cost overruns, and failed trials. Revalia is addressing this breakdown by delivering on-demand access to functional human data through its Human Data Trials - rigorous testing conducted on perfused, human organs maintained under clinical conditions.
"The old model of drug development is broken - decade-long timelines, 90% failure rates, and billion-dollar costs are no longer sustainable," said Greg Tietjen, co-founder and CEO of Revalia Bio. "We're building a new paradigm that allows us to transform the loss of one patient into the future of human-centered development - a new model grounded in real human data, not approximations," he added.
Revalia enables biotech and pharmaceutical companies to evaluate new therapies using data from real human organs, revived and sustained through proprietary perfusion technology. These organs are donated with informed consent from the families and would otherwise be unusable for clinical transplant. This approach improves the accuracy of preclinical data, reduces development costs, and eliminates risks to living patients.
"We see every donated organ as a legacy," said Kourosh Saeb-Parsy, Chief Medical Officer at Revalia Bio. "Our job is to turn that gift into progress-not just for one trial, but for a new opportunity for developing life-saving medicines," he added.
The platform unifies data from perfused human organs, donor medical histories, and high-resolution digital analytics through its Human Data Stack, delivering deeply translatable insight across discovery, safety, biodistribution, and efficacy. Through partnerships with academic medical centers and organ procurement organizations, Revalia repurposes donated, non-transplantable organs into powerful research systems that accelerate therapeutic discovery. By plugging in with existing transplant infrastructure, Revalia has enabled donors who would otherwise not be able to donate for transplantation to donate their organs for research. Donors and their families can leave a lasting legacy by contributing to the next generation of curative therapies.
Since launching commercially in 2023, Revalia has quadrupled its revenue and recently signed two of the world's top 10 pharmaceutical companies as customers. It also developed a first-of-its-kind human lung cancer model in partnership with LifeShare of Oklahoma and achieved key breakthroughs in organ perfusion, including a four-day kidney protocol.
"The ability to generate high-fidelity, human-specific data at scale is one of the most important advancements in biomedicine today," said Brian Wilcove, Managing Partner at America's Frontier Fund. "Revalia's platform has the potential to not only transform clinical trials, but to strengthen national health resilience," he added.
Revalia was founded by a multidisciplinary team of scientists, entrepreneurs, and operators. CEO Greg Tietjen, former tenure track professor at Yale, ran a renowned academic lab that was a world-leader in perfusion science. With a PhD in human organ perfusion from the University of Cambridge, Jenna DiRito brings deep experience in human organ research infrastructure. Kourosh Saeb-Parsy, Peter Buniak, and Helen Hughes add clinical and operational depth to the team. Milad Alucozai, a neuroscientist and first investor, joined the company to help scale it.
"This is about creating a new foundation for medicine, one built on human data, not animal models," said Ben Yu, Managing Partner at Sierra Ventures. "That shift will change not just how we develop treatments, but how we understand biology itself," he added.
Looking ahead, Revalia aims to serve as the foundational infrastructure for Human-Centered Drug Development - offering the insights, systems, and data needed to shift drug development away from animal models and toward truly human-first decision-making. The company's long-term vision is to provide the infrastructure and insight needed to shift drug development to truly human-centered systems that are grounded in the real world problems care providers face every day.
For more information, visit https://revaliabio.com.
Media Contact:
[email protected]
SOURCE: Revalia Bio
View the original press release on ACCESS Newswire
W.Morales--AT



