
-
 Australia bans under-16s from social media in world-first crackdown
Australia bans under-16s from social media in world-first crackdown
-
US Fed appears set for third rate cut despite sharp divides

-
 Veggie 'burgers' at stake in EU negotiations
Veggie 'burgers' at stake in EU negotiations
-
Haitians dance with joy over UNESCO musical listing
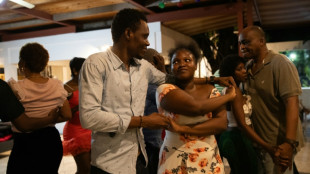
-
 Suspense swirls if Nobel peace laureate will attend ceremony
Suspense swirls if Nobel peace laureate will attend ceremony
-
UK public urged to keep eyes peeled for washed-up bananas

-
 South Korea chip giant SK hynix mulls US stock market listing
South Korea chip giant SK hynix mulls US stock market listing
-
Captain Cummins back in Australia squad for third Ashes Test

-
 NFL Colts to bring 44-year-old QB Rivers out of retirement: reports
NFL Colts to bring 44-year-old QB Rivers out of retirement: reports
-
West Indies 92-2 after being asked to bat in second New Zealand Test

-
 Ruckus in Brazil Congress over bid to reduce Bolsonaro jail term
Ruckus in Brazil Congress over bid to reduce Bolsonaro jail term
-
ExxonMobil slows low-carbon investment push through 2030

-
 Liverpool's Slot swerves further Salah talk after late Inter win
Liverpool's Slot swerves further Salah talk after late Inter win
-
Maresca concerned as Atalanta fight back to beat Chelsea

-
 Liverpool edge Inter in Champions League as Chelsea lose in Italy
Liverpool edge Inter in Champions League as Chelsea lose in Italy
-
Spurs sink Slavia Prague to boost last-16 bid in front of Son

-
 Arsenal ensure Women's Champions League play-off berth
Arsenal ensure Women's Champions League play-off berth
-
Late penalty drama helps Liverpool defy Salah crisis at angry Inter

-
 Canada launches billion dollar plan to recruit top researchers
Canada launches billion dollar plan to recruit top researchers
-
Liverpool defy Salah crisis by beating Inter Milan in Champions League

-
 Honduran leader alleges vote tampering, US interference
Honduran leader alleges vote tampering, US interference
-
De Ketelaere inspires Atalanta fightback to beat Chelsea

-
 Kounde double helps Barcelona claim Frankfurt comeback win
Kounde double helps Barcelona claim Frankfurt comeback win
-
US Supreme Court weighs campaign finance case

-
 Zelensky says ready to hold Ukraine elections, with US help
Zelensky says ready to hold Ukraine elections, with US help
-
Autistic Scottish artist Nnena Kalu smashes Turner Prize 'glass ceiling'

-
 Trump slams 'decaying' and 'weak' Europe
Trump slams 'decaying' and 'weak' Europe
-
Injury-hit Arsenal in 'dangerous circle' but Arteta defends training methods

-
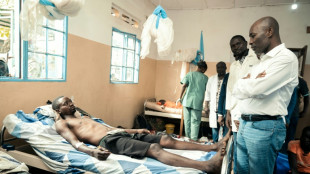 Thousands flee DR Congo fighting as M23 enters key city
Thousands flee DR Congo fighting as M23 enters key city
-
Karl and Gnabry spark Bayern to comeback win over Sporting

-
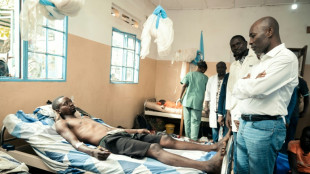 Thousands flee DR Congo fighting as M23 closes on key city
Thousands flee DR Congo fighting as M23 closes on key city
-
Zelensky says ready to hold Ukraine elections

-
 Indigenous artifacts returned by Vatican unveiled in Canada
Indigenous artifacts returned by Vatican unveiled in Canada
-
Ivory Coast recall Zaha for AFCON title defence

-
 Communist vs Catholic - Chile prepares to choose a new president
Communist vs Catholic - Chile prepares to choose a new president
-
Trump's FIFA peace prize breached neutrality, claims rights group

-
 NHL 'optimistic' about Olympic rink but could pull out
NHL 'optimistic' about Olympic rink but could pull out
-
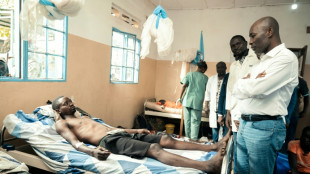
Thousands reported to have fled DR Congo fighting as M23 closes on key city
-
 Three face German court on Russia spying charges
Three face German court on Russia spying charges
-
Amy Winehouse's father sues star's friends for auctioning her clothes

-
 Woltemade's 'British humour' helped him fit in at Newcastle - Howe
Woltemade's 'British humour' helped him fit in at Newcastle - Howe
-
UK trial opens in dispute over Jimi Hendrix recordings

-
 Pandya blitz helps India thrash South Africa in T20 opener
Pandya blitz helps India thrash South Africa in T20 opener
-
Zelensky says will send US revised plan to end Ukraine war

-
 Nobel event cancellation raises questions over Machado's whereabouts
Nobel event cancellation raises questions over Machado's whereabouts
-
Miami's Messi wins second consecutive MLS MVP award

-
 Trump slams 'decaying' Europe and pushes Ukraine on elections
Trump slams 'decaying' Europe and pushes Ukraine on elections
-
TotalEnergies in deal for Namibia offshore oil field

-
 Jesus added to Arsenal's Champions League squad
Jesus added to Arsenal's Champions League squad
-
Red Bull part ways with influential advisor Marko


MygoGenesis Releases White Paper Detailing aLAL(TM) - a Sustainable, Lab-Grown Amebocyte Lysate Designed for Reliable Endotoxin Testing
New Data Describe Lab-Grown Amebocytes (aLAL™) That Aim to Match Native LAL Performance While Solving Supply, Cost and Sustainability Constraints
GERMANTOWN, MARYLAND / ACCESS Newswire / September 4, 2025 / MygoGenesis, a biotechnology company pioneering cell-origin materials through its Mygotic Process™, today announced the release of a new white paper, "Advances in Using Sustainably Generated Amebocytes to Ensure Affordable and Effective Endotoxin Testing." The paper presents scientific and operational evidence supporting aLAL™ - a lysate produced from lab-grown amebocytes - as a sustainable path to high-quality bacterial endotoxin testing.

Harvesting the Blood of Horseshoe Crabs
More Than 1M Horseshoe Crabs are Harvested in the U.S. Each Year for Biomedical Testing
For over 50 years, Limulus Amebocyte Lysate (LAL) from horseshoe crabs has been the global standard for safeguarding injectables, biologics, and medical devices. However, dependence on crab harvesting has introduced ecological strain, supply volatility, and batch variability - pressures that drive up costs and risk. Recombinant approaches have helped, but practical limitations (including low endotoxin recovery and matrix effects) remain.
"MygoGenesis' mission is to unlock ethical, scalable biology that accelerates innovation without compromising the planet," said Deborah Zimmermann, President and CEO of MygoGenesis. "aLAL™ represents a breakthrough: lab-grown amebocytes designed to retain the broad, native cascade of LAL while delivering the consistency, supply assurance, and affordability industry needs. We believe this platform can catalyze better safety testing across pharma, medical devices, water quality, and beyond."
Key points from the white paper include:
A proprietary approach to producing lab-grown amebocytes and formulating aLAL™ to support gel-clot, turbidimetric, and chromogenic assays.
Comparative results indicating comparable detection across a range of EU concentrations versus traditional LAL, alongside immunochemical evidence of cascade parity (e.g., Factor C light-chain).
A roadmap for validation under established pharmacopoeial and quality-system expectations to facilitate adoption.
The potential to de-risk global supply chains, reduce batch-to-batch variability, and lower total testing costs.
"This is a step-change," said Daniel Kilbank, founder and Chief Scientific Officer of MygoGenesis. "For the first time, we can produce functionally relevant amebocytes at scale - not a single recombinant component, but the multi-factor lysate biology industry relies on. That opens the door to consistent performance without harvesting wild horseshoe crabs, expanding access to high-quality endotoxin testing and enabling entirely new applications."
The white paper is available for download here: [Link coming soon].
About MygoGenesis
MygoGenesis is an innovative biotechnology company developing cell-origin materials via its proprietary Mygotic Process™ to solve supply, sustainability, and performance bottlenecks in life sciences. From aLAL™ (lab-grown amebocytes for endotoxin testing) to future applications in diagnostics, water quality, and consumer product safety, MygoGenesis partners with industry leaders to translate breakthroughs into real-world impact. Learn more at mygogenesis.com.
About AmeboGenesis
AmeboGenesis, a division of MygoGenesis, focuses on sustainable amebocyte biology for quality-critical testing. By replacing wild-harvest horseshoe crabs with lab-grown amebocytes, AmeboGenesis seeks to stabilize supply, reduce ecological impact, and improve reproducibility for global endotoxin testing workflows. Visit amebogenesis.com.
Contact Information
Deborah Zimmermann
President & CEO
[email protected]
Eric Tulin
Corporate Communications
[email protected]
(844)327-4953 ext: 707
SOURCE: AmeboGenesis, LLC
View the original press release on ACCESS Newswire
H.Thompson--AT



