-
 Olympic Games in northern Italy have German twist
Olympic Games in northern Italy have German twist
-
Bad Bunny: the Puerto Rican phenom on top of the music world

-
 Snapchat blocks 415,000 underage accounts in Australia
Snapchat blocks 415,000 underage accounts in Australia
-
At Grammys, 'ICE out' message loud and clear

-
 Dalai Lama's 'gratitude' at first Grammy win
Dalai Lama's 'gratitude' at first Grammy win
-
Bad Bunny makes Grammys history with Album of the Year win

-
 Stocks, oil, precious metals plunge on volatile start to the week
Stocks, oil, precious metals plunge on volatile start to the week
-
Steven Spielberg earns coveted EGOT status with Grammy win

-
 Knicks boost win streak to six by beating LeBron's Lakers
Knicks boost win streak to six by beating LeBron's Lakers
-
Kendrick Lamar, Bad Bunny, Lady Gaga triumph at Grammys

-
 Japan says rare earth found in sediment retrieved on deep-sea mission
Japan says rare earth found in sediment retrieved on deep-sea mission
-
San Siro prepares for last dance with Winter Olympics' opening ceremony

-
 France great Benazzi relishing 'genius' Dupont's Six Nations return
France great Benazzi relishing 'genius' Dupont's Six Nations return
-
Grammy red carpet: black and white, barely there and no ICE

-
 Oil tumbles on Iran hopes, precious metals hit by stronger dollar
Oil tumbles on Iran hopes, precious metals hit by stronger dollar
-
South Korea football bosses in talks to avert Women's Asian Cup boycott

-
 Level playing field? Tech at forefront of US immigration fight
Level playing field? Tech at forefront of US immigration fight
-
British singer Olivia Dean wins Best New Artist Grammy

-
 Hatred of losing drives relentless Alcaraz to tennis history
Hatred of losing drives relentless Alcaraz to tennis history
-
Kendrick Lamar, Bad Bunny, Lady Gaga win early at Grammys

-
 Surging euro presents new headache for ECB
Surging euro presents new headache for ECB
-
Djokovic hints at retirement as time seeps away on history bid

-
 US talking deal with 'highest people' in Cuba: Trump
US talking deal with 'highest people' in Cuba: Trump
-
UK ex-ambassador quits Labour over new reports of Epstein links

-
 Trump says closing Kennedy Center arts complex for two years
Trump says closing Kennedy Center arts complex for two years
-
Reigning world champs Tinch, Hocker among Millrose winners

-
 Venezuelan activist ends '1,675 days' of suffering in prison
Venezuelan activist ends '1,675 days' of suffering in prison
-
Real Madrid scrape win over Rayo, Athletic claim derby draw

-
 PSG beat Strasbourg after Hakimi red to retake top spot in Ligue 1
PSG beat Strasbourg after Hakimi red to retake top spot in Ligue 1
-
NFL Cardinals hire Rams' assistant LaFleur as head coach

-
 Arsenal scoop $2m prize for winning FIFA Women's Champions Cup
Arsenal scoop $2m prize for winning FIFA Women's Champions Cup
-
Atletico agree deal to sign Lookman from Atalanta

-
 Real Madrid's Bellingham set for month out with hamstring injury
Real Madrid's Bellingham set for month out with hamstring injury
-
Man City won't surrender in title race: Guardiola

-
 Korda captures weather-shortened LPGA season opener
Korda captures weather-shortened LPGA season opener
-
Czechs rally to back president locking horns with government

-
 Prominent Venezuelan activist released after over four years in jail
Prominent Venezuelan activist released after over four years in jail
-
Emery riled by 'unfair' VAR call as Villa's title hopes fade

-
 Guirassy double helps Dortmund move six points behind Bayern
Guirassy double helps Dortmund move six points behind Bayern
-
Nigeria's president pays tribute to Fela Kuti after Grammys Award

-
 Inter eight clear after win at Cremonese marred by fans' flare flinging
Inter eight clear after win at Cremonese marred by fans' flare flinging
-
England underline World Cup
credentials with series win over Sri Lanka

-
 Guirassy brace helps Dortmund move six behind Bayern
Guirassy brace helps Dortmund move six behind Bayern
-
Man City held by Solanke stunner, Sesko delivers 'best feeling' for Man Utd

-
 'Send Help' debuts atop N.America box office
'Send Help' debuts atop N.America box office
-
Ukraine war talks delayed to Wednesday, says Zelensky

-
 Iguanas fall from trees in Florida as icy weather bites southern US
Iguanas fall from trees in Florida as icy weather bites southern US
-
Carrick revels in 'best feeling' after Man Utd leave it late

-
 Olympic chiefs admit 'still work to do' on main ice hockey venue
Olympic chiefs admit 'still work to do' on main ice hockey venue
-
Pope says Winter Olympics 'rekindle hope' for world peace


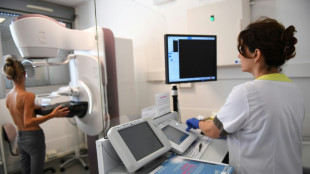
US becomes first country to approve RSV vaccine
The United States on Wednesday approved the world's first vaccine for the Respiratory Syncytial Virus (RSV), the culmination of a decades-long hunt to protect vulnerable people from the common illness.
Drugmaker GSK's Arexvy was green-lighted for adults aged 60 and older, with similar shots from other makers including Pfizer and Moderna expected to follow soon.
"Today's approval of the first RSV vaccine is an important public health achievement to prevent a disease which can be life-threatening," said senior US Food and Drug Administration (FDA) official Peter Marks in a statement.
The decision "marks a turning point in our effort to reduce the significant burden of RSV," added Tony Wood, GSK's chief scientific officer.
RSV is a common virus that normally causes mild, cold-like symptoms, but can be serious for infants and the elderly, as well as those with weak immune systems and underlying conditions.
In severe cases it can cause pneumonia and bronchiolitis, an inflammation of the small airways deep inside the lungs.
According to the US Centers for Disease Control and Prevention, RSV leads to approximately 60,000 to 120,000 hospitalizations and 6,000 to 10,000 deaths among adults 65 years of age and older.
Awareness of the disease has increased in recent years, in part because of the strain it has placed on hospital systems over the last two winters.
Rates of RSV and flu fell during Covid-19 lockdowns, but surged when restrictions were lifted, with young children hit hard.
Pharmaceutical companies have been chasing an RSV vaccine for years. Given recent successful breakthroughs in the sector, analysts predict the market could be worth over $10 billion in the next decade, according to reports.
- More vaccines on way -
GSK's vaccine contains a "subunit" or part of the virus to help train the immune system should it encounter the real thing.
It was approved based on a study of 25,000 people aged 60 and older that showed a single dose was 83 percent effective against disease caused by RSV, and more than 94 percent effective against severe disease.
Researchers will continue to follow volunteers in the study to assess the duration of protection as well as the safety and efficacy of more doses.
The most common side effects included injection site pain, fatigue, muscle pain, headaches and joint stiffness.
An irregular heartbeat was a less common side effect, occurring in 10 participants who received Arexvy and four participants who received placebo.
Safety issues were also found in two other studies of the drug involving approximately 2,500 people aged 60 and up. In one of these studies, two volunteers developed a rare type of inflammation that affects the brain and spinal cord, and one of them died.
In the other study, one participant developed Guillain-Barre syndrome, in which the immune system damages nerve cells, causing muscle weakness and sometimes paralysis.
GSK's Arexvy was recommended for approval last week by the European Union's drug watchdog, the European Medicines Agency, whose positive opinions are normally formally followed by approval from the European Commission.
Pfizer has said that it expects a decision from the FDA in May for its own RSV vaccine, also for those over 60 years old.
In January, Moderna said it hopes its RSV vaccine will be approved and available in time for the Northern Hemisphere's winter later this year.
Several other companies are also developing RSV vaccines.
Last year, the EU approved a preventative antibody treatment against RSV, developed by British-Swedish pharmaceutical firm AstraZeneca and France's Sanofi, which confers temporary protection.
A.Ruiz--AT