
-
 Chile president-elect dials down right-wing rhetoric, vows unity
Chile president-elect dials down right-wing rhetoric, vows unity
-
Five Rob Reiner films that rocked, romanced and riveted

-
 Rob Reiner: Hollywood giant and political activist
Rob Reiner: Hollywood giant and political activist
-
Observers say Honduran election fair, but urge faster count

-
 Europe proposes Ukraine peace force as Zelensky hails 'real progress' with US
Europe proposes Ukraine peace force as Zelensky hails 'real progress' with US
-
Trump condemned for saying critical filmmaker brought on own murder

-
 US military to use Trinidad airports, on Venezuela's doorstep
US military to use Trinidad airports, on Venezuela's doorstep
-
Daughter warns China not to make Jimmy Lai a 'martyr'

-
 UK defence chief says 'whole nation' must meet global threats
UK defence chief says 'whole nation' must meet global threats
-
Rob Reiner's death: what we know

-
 Zelensky hails 'real progress' in Berlin talks with Trump envoys
Zelensky hails 'real progress' in Berlin talks with Trump envoys
-
Toulouse handed two-point deduction for salary cap breach

-
 Son arrested for murder of movie director Rob Reiner and wife
Son arrested for murder of movie director Rob Reiner and wife
-
Stock market optimism returns after tech selloff but Wall Street wobbles

-
 Clarke warns Scotland fans over sky-high World Cup prices
Clarke warns Scotland fans over sky-high World Cup prices
-
In Israel, Sydney attack casts shadow over Hanukkah

-
 Son arrested after Rob Reiner and wife found dead: US media
Son arrested after Rob Reiner and wife found dead: US media
-
Athletes to stay in pop-up cabins in the woods at Winter Olympics

-
 England seek their own Bradman in bid for historic Ashes comeback
England seek their own Bradman in bid for historic Ashes comeback
-
Decades after Bosman, football's transfer war rages on

-
 Ukraine hails 'real progress' in Zelensky's talks with US envoys
Ukraine hails 'real progress' in Zelensky's talks with US envoys
-
Nobel winner Machado suffered vertebra fracture leaving Venezuela

-
 Stock market optimism returns after tech sell-off
Stock market optimism returns after tech sell-off
-
Iran Nobel winner unwell after 'violent' arrest: supporters

-
 Police suspect murder in deaths of Hollywood giant Rob Reiner and wife
Police suspect murder in deaths of Hollywood giant Rob Reiner and wife
-
'Angry' Louvre workers' strike shuts out thousands of tourists

-
 EU faces key summit on using Russian assets for Ukraine
EU faces key summit on using Russian assets for Ukraine
-
Maresca committed to Chelsea despite outburst

-
 Trapped, starving and afraid in besieged Sudan city
Trapped, starving and afraid in besieged Sudan city
-
Showdown looms as EU-Mercosur deal nears finish line

-
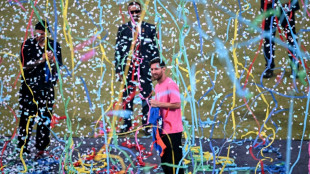 Messi mania peaks in India's pollution-hit capital
Messi mania peaks in India's pollution-hit capital
-
Wales captains Morgan and Lake sign for Gloucester

-
 Serbian minister indicted over Kushner-linked hotel plan
Serbian minister indicted over Kushner-linked hotel plan
-
Eurovision 2026 will feature 35 countries: organisers

-
 Cambodia says Thailand bombs province home to Angkor temples
Cambodia says Thailand bombs province home to Angkor temples
-
US-Ukrainian talks resume in Berlin with territorial stakes unresolved

-
 Small firms join charge to boost Europe's weapon supplies
Small firms join charge to boost Europe's weapon supplies
-
Driver behind Liverpool football parade 'horror' warned of long jail term

-
 German shipyard, rescued by the state, gets mega deal
German shipyard, rescued by the state, gets mega deal
-
Flash flood kills dozens in Morocco town

-
 'We are angry': Louvre Museum closed as workers strike
'We are angry': Louvre Museum closed as workers strike
-
Australia to toughen gun laws as it mourns deadly Bondi attack

-
 Stocks diverge ahead of central bank calls, US data
Stocks diverge ahead of central bank calls, US data
-
Wales captain Morgan to join Gloucester

-
 UK pop star Cliff Richard reveals prostate cancer treatment
UK pop star Cliff Richard reveals prostate cancer treatment
-
Mariah Carey to headline Winter Olympics opening ceremony

-
 Indonesia to revoke 22 forestry permits after deadly floods
Indonesia to revoke 22 forestry permits after deadly floods
-
Louvre Museum closed as workers strike

-
 Spain fines Airbnb 64 mn euros for posting banned properties
Spain fines Airbnb 64 mn euros for posting banned properties
-
Japan's only two pandas to be sent back to China

| RBGPF | -4.49% | 77.68 | $ | |
| CMSC | -0.13% | 23.27 | $ | |
| RYCEF | 2.01% | 14.9 | $ | |
| SCS | 0.12% | 16.14 | $ | |
| NGG | 1.47% | 76.05 | $ | |
| GSK | 1.1% | 49.355 | $ | |
| VOD | 1.06% | 12.725 | $ | |
| RELX | 1.7% | 41.08 | $ | |
| AZN | 1.93% | 91.598 | $ | |
| RIO | 0.12% | 75.75 | $ | |
| CMSD | 0.51% | 23.37 | $ | |
| JRI | 0.25% | 13.5999 | $ | |
| BCC | -0.78% | 75.92 | $ | |
| BCE | 0.41% | 23.491 | $ | |
| BTI | 0.91% | 57.625 | $ | |
| BP | -0.1% | 35.225 | $ |

Applied DNA's LineaRx Subsidiary Stands Ready to Support the Reshoring of Drug Development and Manufacturing with U.S.-Produced Synthetic DNA
- Pivot to U.S.-based Supply Chain for Critical Input Materials Completed -
STONY BROOK, NY / ACCESS Newswire / April 21, 2025 / Applied DNA Sciences, Inc. (NASDAQ:APDN) (Applied DNA or the "Company"), a leader in PCR-based DNA technologies, today announced that LineaRx, Inc., the Company's majority-owned subsidiary, has completed a long-term initiative to source critical input materials for its LineaDNA™ and LineaIVT™ platforms from U.S.-based suppliers. The initiative, undertaken in response to the 2024 BIOSECURE Act and customer demand for a U.S.-based supply chain, comes as the biopharmaceutical industry increasingly considers reshoring manufacturing operations to the U.S. in response to potential tariff impacts and to ensure a stable supply chain.
"With the completion of our supply chain pivot, the LineaDNA platform is now engineered for speed, scale, and domestic sourcing. Localized production of our DNA template materials and manufacturing enzymes enables us to better position LineaRx as a trusted and reliable manufacturing partner for developing and producing genetic medicines," stated Clay D. Shorrock, president of LineaRx. "As the largest PCR-based cell-free DNA producer in the United States and with capabilities further enhanced by the recent launch of GMP manufacturing services, we believe we are well-positioned to support increased domestic development and manufacturing of a wide range of genetic medicines, ranging from mRNA/DNA products to cell and gene therapies."
LineaRx utilizes its proprietary PCR-based LineaDNA™ platform to rapidly manufacture high-fidelity, cell-free DNA used in the development and manufacture of genetic medicines in quantities ranging from milligram to multi-gram scale. By leveraging its PCR-based technology, which requires a minimal number of critical input materials, LineaRx has built a robust, U.S.-based supply chain inclusive of DNA template materials and manufacturing enzymes that represent over 75% of its manufacturing cost of goods. Previously, in June 2024, LineaRx concluded manufacturing scale-up for its proprietary RNA polymerase (LineaRNAPTM) with a U.S.-based manufacturer.
About the LineaDNA™ and LineaIVT™ Platforms
The LineaDNA platform is an entirely cell-free DNA production platform founded on Applied DNA's long-standing expertise in the large-scale enzymatic production of DNA. Capable of producing DNA in quantities ranging from milligrams to grams, the LineaDNA platform can produce high-fidelity DNA constructs ranging from 100bp to 20kb in size. The DNA produced via the LineaDNA platform is free of the adventitious DNA sequences found in other sources of DNA, is rapidly scalable, and provides for simple chemical modification of DNA constructs. The LineaIVT platform combines DNA IVT template manufacturing via the LineaDNA platform with a proprietary RNA polymerase, LineaRNAP™, to enable mRNA and sa-mRNA manufacturers to produce what Applied DNA believes to be better mRNA, faster, with advantages over conventional mRNA production, including: 1) the elimination of plasmid DNA as a starting material; 2) the prevention or reduction of double-stranded DNA (dsRNA) contamination; and 3) simplified mRNA production workflows.
About Applied DNA Sciences
Applied DNA Sciences is a biotechnology company developing technologies to produce and detect deoxyribonucleic acid ("DNA"). Using the polymerase chain reaction ("PCR") to enable both the production and detection of DNA, we operate in two business markets: (i) the enzymatic manufacture of synthetic DNA for use in the production of nucleic acid-based therapeutics and the development and sale of a proprietary RNA polymerase ("RNAP") for use in the production of mRNA therapeutics; and (ii) the detection of DNA and RNA in molecular diagnostics and genetic testing services.
Visit adnas.com for more information. Follow us on X and LinkedIn.
Forward-Looking Statements
The statements made by Applied DNA in this press release may be "forward-looking" in nature within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934, and the Private Securities Litigation Reform Act of 1995. Forward-looking statements describe Applied DNA's future plans, projections, strategies, and expectations, and are based on assumptions and involve a number of risks and uncertainties, many of which are beyond the control of Applied DNA. Actual results could differ materially from those projected due to its history of net losses, limited financial resources, the unknown future impacts of current and future tariffs and geopolitical agendas, the unknown future demand for its biotherapeutics products and services, the unknown amount of revenues and profits that will result from its LineaIVT™ and or LineaDNA™ platforms, the fact that there has never been clinical trial material and/or commercial drug product produced utilizing the LineaDNA and/or Linea IVT platforms, and various other factors detailed from time to time in Applied DNA's SEC reports and filings, including its Annual Report on Form 10-K filed on December 17, 2024, its Quarterly Report on Form 10-Q filed on February 13, 2025, and other reports it files with the SEC, which are available at www.sec.gov. Applied DNA undertakes no obligation to update publicly any forward-looking statements to reflect new information, events, or circumstances after the date hereof or to reflect the occurrence of unanticipated events, unless otherwise required by law.
Applied DNA Sciences Contact:
Investor Relations contact: Sanjay M. Hurry, 917-733-5573, [email protected]
Web: adnas.com
###
SOURCE: Applied DNA Sciences, Inc.
View the original press release on ACCESS Newswire
Th.Gonzalez--AT
