
-
 Trump says Iran wants deal, US 'armada' larger than in Venezuela raid
Trump says Iran wants deal, US 'armada' larger than in Venezuela raid
-
US Justice Dept releases new batch of documents, images, videos from Epstein files

-
 Four memorable showdowns between Alcaraz and Djokovic
Four memorable showdowns between Alcaraz and Djokovic
-
Russian figure skating prodigy Valieva set for comeback -- but not at Olympics

-
 Barcelona midfielder Lopez agrees contract extension
Barcelona midfielder Lopez agrees contract extension
-
Djokovic says 'keep writing me off' after beating Sinner in late-nighter

-
 US Justice Dept releasing new batch of Epstein files
US Justice Dept releasing new batch of Epstein files
-
South Africa and Israel expel envoys in deepening feud

-
 French eyewear maker in spotlight after presidential showing
French eyewear maker in spotlight after presidential showing
-
Olympic dream 'not over', Vonn says after crash

-
 Brazil's Lula discharged after cataract surgery
Brazil's Lula discharged after cataract surgery
-
US Senate races to limit shutdown fallout as Trump-backed deal stalls

-
 'He probably would've survived': Iran targeting hospitals in crackdown
'He probably would've survived': Iran targeting hospitals in crackdown
-
Djokovic stuns Sinner to set up Australian Open final with Alcaraz

-
 Mateta omitted from Palace squad to face Forest
Mateta omitted from Palace squad to face Forest
-
Gold, silver prices tumble as investors soothed by Trump's Fed pick

-
 Trump attorney general orders arrest of ex-CNN anchor covering protests
Trump attorney general orders arrest of ex-CNN anchor covering protests
-
Djokovic 'pushed to the limit' in stunning late-night Sinner upset

-
 Tunisia's famed blue-and-white village threatened after record rains
Tunisia's famed blue-and-white village threatened after record rains
-
Top EU official voices 'shock' at Minneapolis violence

-
 Kremlin says agreed to halt strikes on Kyiv until Sunday
Kremlin says agreed to halt strikes on Kyiv until Sunday
-
Carrick calls for calm after flying start to Man Utd reign

-
 Djokovic to meet Alcaraz in Melbourne final after five-set marathon
Djokovic to meet Alcaraz in Melbourne final after five-set marathon
-
Italian officials to testify in trial over deadly migrant shipwreck

-
 Iran says defence capabilities 'never' up for negotiation
Iran says defence capabilities 'never' up for negotiation
-
UN appeals for more support for flood-hit Mozambicans

-
 Lijnders urges Man City to pile pressure on Arsenal in title race
Lijnders urges Man City to pile pressure on Arsenal in title race
-
Fulham sign Man City winger Oscar Bobb

-
 Strasbourg's Argentine striker Panichelli sets sights on PSG, World Cup
Strasbourg's Argentine striker Panichelli sets sights on PSG, World Cup
-
Jesus 'made love': Colombian president irks Christians with steamy claim

-
 IAEA board meets over Ukraine nuclear safety concerns
IAEA board meets over Ukraine nuclear safety concerns
-
Eurozone growth beats 2025 forecasts despite Trump woes

-
 Israel to partially reopen Gaza's Rafah crossing on Sunday
Israel to partially reopen Gaza's Rafah crossing on Sunday
-
Dutch PM-elect Jetten says not yet time to talk to Putin

-
 Social media fuels surge in UK men seeking testosterone jabs
Social media fuels surge in UK men seeking testosterone jabs
-
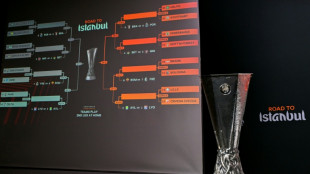
Forest face Fenerbahce, Celtic draw Stuttgart in Europa League play-offs
-
 US speed queen Vonn crashes at Crans-Montana, one week before Olympics
US speed queen Vonn crashes at Crans-Montana, one week before Olympics
-
Trump nominates former US Fed official as next central bank chief

-
 Alcaraz defends controversial timeout after beaten Zverev fumes
Alcaraz defends controversial timeout after beaten Zverev fumes
-
New Dutch government pledges ongoing Ukraine support

-
 Newcastle still coping with fallout from Isak exit, says Howe
Newcastle still coping with fallout from Isak exit, says Howe
-
Chad, France eye economic cooperation as they reset strained ties

-
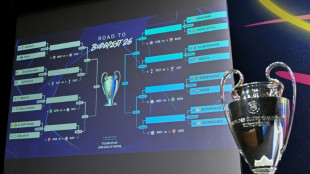 Real Madrid to play Benfica, PSG face Monaco in Champions League play-offs
Real Madrid to play Benfica, PSG face Monaco in Champions League play-offs
-
Everton winger Grealish set to miss rest of season in World Cup blow

-
 Trump brands Minneapolis nurse killed by federal agents an 'agitator'
Trump brands Minneapolis nurse killed by federal agents an 'agitator'
-
Arteta focuses on the positives despite Arsenal stumble

-
 Fijian Drua sign France international back Vakatawa
Fijian Drua sign France international back Vakatawa
-
Kevin Warsh, a former Fed 'hawk' now in tune with Trump

-
 Zverev rails at Alcaraz timeout in 'one of the best battles ever'
Zverev rails at Alcaraz timeout in 'one of the best battles ever'
-
Turkey leads Iran diplomatic push as Trump softens strike threat

| RBGPF | 1.65% | 83.78 | $ | |
| SCS | 0.12% | 16.14 | $ | |
| RYCEF | -2.69% | 16 | $ | |
| CMSC | 0.02% | 23.7 | $ | |
| BCC | -0.97% | 79.4 | $ | |
| BTI | -0.12% | 60.135 | $ | |
| RELX | -1.69% | 35.565 | $ | |
| NGG | -0.81% | 84.37 | $ | |
| GSK | 1.33% | 51.34 | $ | |
| RIO | -4.86% | 90.725 | $ | |
| JRI | 0.32% | 12.997 | $ | |
| CMSD | -0.12% | 24.03 | $ | |
| BCE | -0.18% | 25.44 | $ | |
| VOD | -0.58% | 14.625 | $ | |
| AZN | 0.75% | 93.285 | $ | |
| BP | -1.1% | 37.625 | $ |

LIR Life Sciences Completes Design of Ex Vivo Animal Study to Evaluate Novel Transdermal Agents for Transport of Larger Therapeutic Molecules
VANCOUVER, BC / ACCESS Newswire / January 22, 2026 / LIR Life Sciences Corp. (CSE:SKNY)(Frankfurt:N790, WKN: A41QA9) ("LIR" or the "Company") is pleased to announce the completion of a study design for an ex vivo porcine skin investigation that is designed to evaluate whether novel transdermal agents can enhance the transdermal penetration of therapeutic macromolecules across a wide molecular size range, spanning approximately 5kDa up to antibody scale (~150kDa). In particular, the study will test whether transdermal agents can help much larger medicines move through skin effectively, which could potentially open the door to developing certain therapies without injections.
Working with its scientific partners, LIR has finalized a controlled experimental framework using full thickness porcine skin, which is commonly accepted as one of the most relevant models for human skin permeability. The study is designed to compare transdermal agent-containing formulations with matched controls that contain the same macromolecular payloads without transdermal agents. Penetration depth and distribution will be evaluated using confocal microscopy together with quantitative fluorescence-based measurements collected at predefined time points. These analyses are intended to help determine whether the transdermal agents can meaningfully facilitate the movement of macromolecular therapeutics through the skin to a measurable degree.
Completion of the design phase allows LIR to proceed towards formal study execution, which is expected to occur in Q1, 2026. The results are expected to inform which categories of larger therapeutic molecules may ultimately be compatible with skin applied, needle free delivery strategies.
"Our focus in designing this study was to de risk the science with a disciplined methodology that uses a human relevant skin model and rigorous quantitative analysis. We believe there is real potential here, and are excited to implement the study so we can determine whether the novel transdermal agents deliver the level of penetration we expect, thereby reinforcing the opportunity to pursue a practical needle free approach to delivering larger therapeutics through the skin," said Edward Mills, CEO of LIR Life Sciences.
AboutLIR Life Sciences Corp.
LIR Life Sciences is focused on researching and developing scalable and affordable treatments for obesity using novel drug delivery methods. The company is advancing a transdermal patch and other novel delivery systems that mimic GLP-1, a naturally occurring hormone that helps regulate appetite and blood sugar. These therapies could potentially offer an alternative to injectable drugs. The goal is to improve access, adherence, and cost-efficiency in both developed and emerging markets. LIR Life Sciences aims to address the global burden of obesity with practical solutions based on established compounds and proven science.
ON BEHALF OF LIR LIFE SCIENCES CORP.,
"Dr.Edward Mills,"
Chief Executive Officer
For more information, please contact:
Dr. Edward Mills
Chief Executive Officer
Tel: +1 888 436 7772
Email: [email protected]
Neither the CSE nor its Regulation Services Provider accepts responsibility for the adequacy or accuracy of this news release. No stock exchange, securities commission or other regulatory authority has approved or disapproved the information contained herein.
Cautionary Note Regarding Forward-Looking Information
This news release contains statements and information that, to the extent that they are not historical fact, may constitute "forward-looking information" within the meaning of applicable securities legislation based on current expectations, estimates, forecasts, projections, beliefs and assumptions made by management of the Company. Forward-looking information is generally identified by words such as "believe", "project", "aim", "expect", "anticipate", "estimate", "intend", "strategy", "future", "opportunity", "plan", "may", "should", "will", "would", and similar expressions and, in this news release, includes statements relating to the implementation of the study described herein, the research and development activities of the Company, the financial and business prospects of the Company, its assets and other matters. In particular, forward-looking information includes statements regarding the Company's plans to conduct the study described herein, its transdermal delivery platform, the potential compatibility of the platform with GLP/GIP-based medicines, the anticipated outcomes of preclinical studies, and the potential development of future needle-free metabolic therapies. Although the Company believes that the expectations and assumptions on which such forward- looking information are reasonable, undue reliance should not be placed on the forward-looking information because the Company can give no assurance that it will prove to be correct. Since forward-looking information addresses future events and conditions, by its very nature it involves inherent risks and uncertainties. Many factors could cause actual future events to differ materially from the forward-looking information in this news release. The forward-looking information included in this news release is expressly qualified by this cautionary statement. The forward-looking information contained in this news release is made as of the date hereof and the Company undertakes no obligation to update publicly or revise any forward-looking information, whether as a result of new information, future events or otherwise, unless so required by applicable laws.
SOURCE: Lir Life Sciences Corp.
View the original press release on ACCESS Newswire
A.Moore--AT




