-
 Uganda counting votes amid reports of violence
Uganda counting votes amid reports of violence
-
Stock markets slip with trade deals in focus

-
 Arteta says consistency can fire Arsenal to special season
Arteta says consistency can fire Arsenal to special season
-
Van Nistelrooy to rejoin Dutch coaching team for World Cup

-
 Protected forests under threat in DRC's lucrative mining belt
Protected forests under threat in DRC's lucrative mining belt
-
Iran protest movement subsides in face of 'brutal' crackdown
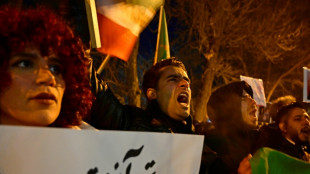
-
 Myanmar tells ICJ Rohingya genocide claims 'unsubstantiated'
Myanmar tells ICJ Rohingya genocide claims 'unsubstantiated'
-
Slot 'happy' to welcome Salah back at Liverpool after AFCON

-
 Experts cast doubt on Burkina Faso's 'foiled coup'
Experts cast doubt on Burkina Faso's 'foiled coup'
-
France says parliament approval of budget 'impossible'

-
 South Korean ex-leader jailed for 5 years in first martial law verdict
South Korean ex-leader jailed for 5 years in first martial law verdict
-
The murky fates of South Korean presidents

-
 Spanish singer Julio Iglesias says abuse allegations 'absolutely false'
Spanish singer Julio Iglesias says abuse allegations 'absolutely false'
-
'Hobbit houses' that might just save a Moldovan village

-
 Verstappen motivated as Red Bull braces for F1 changes
Verstappen motivated as Red Bull braces for F1 changes
-
Fraudsters flee Cambodia's 'scam city' after accused boss taken down

-
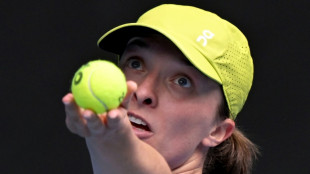 Career Grand Slam 'a dream' for Swiatek but not key focus in Melbourne
Career Grand Slam 'a dream' for Swiatek but not key focus in Melbourne
-
Malnutrition having 'harrowing' impact on Afghan women: WFP

-
 Afghan mothers seek hospital help for malnourished children
Afghan mothers seek hospital help for malnourished children
-
Coaches Regragui and Thiaw have suffered AFCON final heartbreak

-
 US strikes deal with Taiwan to cut tariffs, boost chip investment
US strikes deal with Taiwan to cut tariffs, boost chip investment
-
Anisimova targets third Slam final in a row after breakout year

-
 Canada's Carney hails 'strategic partnership' in talks with Xi
Canada's Carney hails 'strategic partnership' in talks with Xi
-
Japan and US agree to expand cooperation on missiles, military drills

-
 China's 2025 economic growth likely slowest in decades: analysts
China's 2025 economic growth likely slowest in decades: analysts
-
Defending champ Nick Taylor tied for lead at Sony Open

-
 Five memorable Africa Cup of Nations finals
Five memorable Africa Cup of Nations finals
-
Asian stocks mixed after bumper TSMC results

-
 Sabalenka insists Australian Open not just between her and Swiatek
Sabalenka insists Australian Open not just between her and Swiatek
-
Surveillance, harassment and bribes: everyday life for migrants in Russia

-
 Libyan filmmaker fights for cinema revival
Libyan filmmaker fights for cinema revival
-
Afghan man goes on trial over deadly Munich car-ramming

-
 NATO chief's tactic on Trump's Greenland threats? Change topic
NATO chief's tactic on Trump's Greenland threats? Change topic
-
'Was hoping for more': Trump support slips one year in

-
 McIntosh, Marchand grab medley victories in Austin, Ledecky wins again
McIntosh, Marchand grab medley victories in Austin, Ledecky wins again
-
Sinner says doping scandal made him stronger

-
 Vietnam leader seeks more power at party congress
Vietnam leader seeks more power at party congress
-
As Trump turns screws, how long can Europe play nice?

-
 Carrick given Manchester derby baptism of fire, Frank in the firing line
Carrick given Manchester derby baptism of fire, Frank in the firing line
-
Trump announces 'board of peace' formed for Gaza

-
 Sam Darnold: the 'old soul' QB tipped to win Super Bowl
Sam Darnold: the 'old soul' QB tipped to win Super Bowl
-
One year on, it's all about Trump. But for how long?

-
 Australian snowboarder Brockhoff quits ahead of Winter Olympics
Australian snowboarder Brockhoff quits ahead of Winter Olympics
-
Bills battle Broncos as Allen eyes Super Bowl

-
 YouWare Launches YouBase to Support Full-Stack Application Development
YouWare Launches YouBase to Support Full-Stack Application Development
-
Mosaic Issues Market Update And Preliminary Fourth Quarter 2025 Sales Results

-
 BioLargo to Present at Sidoti's Micro-Cap Virtual Conference on Thursday January 22nd at 1pm EST
BioLargo to Present at Sidoti's Micro-Cap Virtual Conference on Thursday January 22nd at 1pm EST
-
CelLBxHealth PLC Announces Preliminary Fourth Quarter 2025 Financial Results

-
 Magic rally to top Grizzlies in NBA Berlin game
Magic rally to top Grizzlies in NBA Berlin game
-
Venezuela's Machado says she 'presented' Trump with Nobel medal


LIR Life Sciences Launches Comparative Animal Study to Advance Transdermal Delivery of Second Generation GLP/GIP-based Obesity Therapies
VANCOUVER, BC / ACCESS Newswire / January 15, 2026 / LIR Life Sciences Corp. (CSE:SKNY)(OTC PINK:BBCMF)(Frankfurt:N790, WKN:A41QA9) ("LIR" or the "Company")is pleased to announce the launch of a controlled comparative animal study designed to evaluate cell-penetrating peptide (CPP) mediated, needle-free transdermal delivery of second generation GLP/GIP-based obesity therapies. This in vivo study represents an important next step towards advancing LIR's transdermal platform from design-stage planning into functional testing in a small animal model.
Working with its scientific collaborators, LIR will assess whether a CPP-enabled, skin-applied formulation of a representative second generation GLP/GIP-based obesity therapy can achieve meaningful biological activity when delivered through the skin. Study animals will receive either a topical CPP formulation or a standard subcutaneous injection, followed by a controlled glucose challenge. Blood glucose levels will be measured over time to compare how effectively each route of administration supports glucose control.
The study will use a standard glucose tolerance test, a well-established method in metabolic research and clinical practice.1 Incretin-based therapies that are working as intended have been shown to help keep the blood sugar curve lower and flatter after the glucose load.2 If the CPP-enabled transdermal formulation is found to deliver sufficient drug into circulation, LIR expects that animals treated via the skin should show more stable glucose profiles.
By focusing on GLP/GIP-based incretin therapies as a class, rather than a single branded product, the study is intended to probe the broader applicability of the CPP-enabled transdermal platform. The resulting data are expected to help define where needle-free delivery may match or approach injectable performance and to guide selection of the most promising molecules for further development and potential IND-enabling work.
These efforts align with LIR's objective to create patient-friendly, scalable alternatives to injectable incretin therapies for obesity and related metabolic conditions. Needle-free, skin-applied treatments could simplify administration, improve adherence, and reduce treatment burden for patients who might otherwise require frequent injections for long-term weight and glucose management.
"What matters most about this study is the ability to explore a delivery pathway with potential relevance across a whole class of incretin therapies, rather than focusing on one molecule alone. Demonstrating glucose control from a skin-applied formulation would validate a tangible therapeutic pathway with the potential to streamline development across multiple GLP/GIP-based drugs," said Edward Mills, CEO of LIR Life Sciences.
The Company also announces that it has engaged the services of ICP Securities Inc. ("ICP") (business address: 251 Queens Quay East, Suite 204, Toronto, ON, M5A 0X3; email: [email protected]; telephone: +16478738519; and contact name: David Campbell) to provide automated market making services, including use of its proprietary algorithm, ICP Premium™, in compliance with the policies and guidelines of the CSE Exchange and other applicable legislation. ICP will be paid a monthly fee of C$7,500, plus applicable taxes. The agreement between the Company and ICP was signed with a start date of January 13, 2026 and is for four (4) months (the "Initial Term") and shall be automatically renewed for subsequent one (1) month terms (each month called an "Additional Term") unless either party provides at least thirty (30) days written notice prior to the end of the Initial Term or an Additional Term, as applicable. There are no performance factors contained in the agreement and no stock options or other compensation in connection with the engagement. ICP and its clients may acquire an interest in the securities of the Company in the future.
ICP is an arm's length party to the Company. ICP's market making activity will be primarily to correct temporary imbalances in the supply and demand of the Company's shares. ICP will be responsible for the costs it incurs in buying and selling the Company's shares, and no third party will be providing funds or securities for the market making activities.
AboutLIR Life Sciences Corp.
LIR Life Sciences is focused on researching and developing scalable and affordable treatments for obesity using novel drug delivery methods. The company is advancing a transdermal patch and other novel delivery systems that mimic GLP-1, a naturally occurring hormone that helps regulate appetite and blood sugar. These therapies could potentially offer an alternative to injectable drugs. The goal is to improve access, adherence, and cost-efficiency in both developed and emerging markets. LIR Life Sciences aims to address the global burden of obesity with practical solutions based on established compounds and proven science.
ON BEHALF OF LIR LIFE SCIENCES CORP.,
"Dr.Edward Mills,"
Chief Executive Officer
For more information, please contact:
Dr. Edward Mills
Chief Executive Officer
Tel: +1 888 436 7772
Email: [email protected]
Neither the CSE nor its Regulation Services Provider accepts responsibility for the adequacy or accuracy of this news release. No stock exchange, securities commission or other regulatory authority has approved or disapproved the information contained herein.
Cautionary Note Regarding Forward-Looking Information
This news release contains statements and information that, to the extent that they are not historical fact, may constitute "forward-looking information" within the meaning of applicable securities legislation based on current expectations, estimates, forecasts, projections, beliefs and assumptions made by management of the Company. Forward-looking information is generally identified by words such as "believe", "project", "aim", "expect", "anticipate", "estimate", "intend", "strategy", "future", "opportunity", "plan", "may", "should", "will", "would", and similar expressions and, in this news release, includes statements relating to the research and development activities of the Company, the financial and business prospects of the Company, its assets and other matters. In particular, forward-looking information includes statements regarding the Company's R&D plans, transdermal delivery platform, the potential compatibility of the platform with GLP/GIP-based medicines, the anticipated outcomes of preclinical studies, and the potential development of future needle-free metabolic therapies. Although the Company believes that the expectations and assumptions on which such forward- looking information are reasonable, undue reliance should not be placed on the forward-looking information because the Company can give no assurance that it will prove to be correct. Since forward-looking information addresses future events and conditions, by its very nature it involves inherent risks and uncertainties. Many factors could cause actual future events to differ materially from the forward-looking information in this news release. The forward-looking information included in this news release is expressly qualified by this cautionary statement. The forward-looking information contained in this news release is made as of the date hereof and the Company undertakes no obligation to update publicly or revise any forward-looking information, whether as a result of new information, future events or otherwise, unless so required by applicable laws.
1 American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes. Diabetes Care.
2 Nauck MA, Meier JJ. Incretin hormones: Their role in health and disease. Diabetes Obes. Metabol. 2018 Feb:20 Suppl 1:5-21.
SOURCE: Lir Life Sciences Corp.
View the original press release on ACCESS Newswire
E.Rodriguez--AT




