-
 Honduras begins partial vote recount in Trump-dominated election
Honduras begins partial vote recount in Trump-dominated election
-
Nike shares slump as China struggles continue

-
 Hundreds swim, float at Bondi Beach to honour shooting victims
Hundreds swim, float at Bondi Beach to honour shooting victims
-
Crunch time for EU leaders on tapping Russian assets for Ukraine

-
 Pope replaces New York's pro-Trump Cardinal with pro-migrant Chicagoan
Pope replaces New York's pro-Trump Cardinal with pro-migrant Chicagoan
-
Trump orders marijuana reclassified as less dangerous drug

-
 Rams ace Nacua apologizes over 'antisemitic' gesture furor
Rams ace Nacua apologizes over 'antisemitic' gesture furor
-
McIlroy wins BBC sports personality award for 2025 heroics

-
 Napoli beat Milan in Italian Super Cup semi-final
Napoli beat Milan in Italian Super Cup semi-final
-
Violence erupts in Bangladesh after wounded youth leader dies

-
 EU-Mercosur deal delayed as farmers stage Brussels show of force
EU-Mercosur deal delayed as farmers stage Brussels show of force
-
US hosting new Gaza talks to push next phase of deal

-
 Chicago Bears mulling Indiana home over public funding standoff
Chicago Bears mulling Indiana home over public funding standoff
-
Trump renames Kennedy arts center after himself

-
 Trump rebrands housing supplement as $1,776 bonuses for US troops
Trump rebrands housing supplement as $1,776 bonuses for US troops
-
Harrison Ford to get lifetime acting award

-
 Trump health chief seeks to bar trans youth from gender-affirming care
Trump health chief seeks to bar trans youth from gender-affirming care
-
Argentine unions in the street over Milei labor reforms

-
 Trump signs order reclassifying marijuana as less dangerous
Trump signs order reclassifying marijuana as less dangerous
-
Famed Kennedy arts center to be renamed 'Trump-Kennedy Center'

-
 US accuses S.Africa of harassing US officials working with Afrikaners
US accuses S.Africa of harassing US officials working with Afrikaners
-
Brazil open to EU-Mercosur deal delay as farmers protest in Brussels

-
 Wounded Bangladesh youth leader dies in Singapore hospital
Wounded Bangladesh youth leader dies in Singapore hospital
-
New photo dump fuels Capitol Hill push on Epstein files release

-
 Brazil, Mexico seek to defuse US-Venezuela crisis
Brazil, Mexico seek to defuse US-Venezuela crisis
-
Assange files complaint against Nobel Foundation over Machado win

-
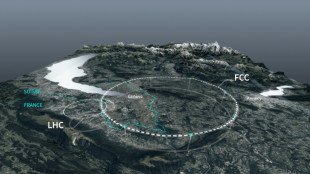 Private donors pledge $1 bn for CERN particle accelerator
Private donors pledge $1 bn for CERN particle accelerator
-
Russian court orders Austrian bank Raiffeisen to pay compensation

-
 US, Qatar, Turkey, Egypt to hold Gaza talks in Miami
US, Qatar, Turkey, Egypt to hold Gaza talks in Miami
-
Lula open to mediate between US, Venezuela to 'avoid armed conflict'

-
 Brussels farmer protest turns ugly as EU-Mercosur deal teeters
Brussels farmer protest turns ugly as EU-Mercosur deal teeters
-
US imposes sanctions on two more ICC judges for Israel probe

-
 US accuses S. Africa of harassing US officials working with Afrikaners
US accuses S. Africa of harassing US officials working with Afrikaners
-
ECB holds rates as Lagarde stresses heightened uncertainty

-
 Trump Media announces merger with fusion power company
Trump Media announces merger with fusion power company
-
Stocks rise as US inflation cools, tech stocks bounce

-
 Zelensky presses EU to tap Russian assets at crunch summit
Zelensky presses EU to tap Russian assets at crunch summit
-
Pope replaces New York's Cardinal Dolan with pro-migrant bishop

-
 Odermatt takes foggy downhill for 50th World Cup win
Odermatt takes foggy downhill for 50th World Cup win
-
France exonerates women convicted over abortions before legalisation

-
 UK teachers to tackle misogyny in classroom
UK teachers to tackle misogyny in classroom
-
Historic Afghan cinema torn down for a mall

-
 US consumer inflation cools unexpectedly in November
US consumer inflation cools unexpectedly in November
-
Danish 'ghetto' residents upbeat after EU court ruling

-
 ECB holds rates but debate swirls over future
ECB holds rates but debate swirls over future
-
Pope replaces New York's Cardinal Timothy Dolan with little-known bishop

-
 Bank of England cuts interest rate after UK inflation slides
Bank of England cuts interest rate after UK inflation slides
-
Have Iran's authorities given up on the mandatory hijab?

-
 Spain to buy 100 military helicopters from Airbus
Spain to buy 100 military helicopters from Airbus
-
US strike on alleged drug boat in Pacific kills four


Regentis Biomaterials Granted New U.S. Patent for its Off-the-Shelf Regenerative Cartilage Repair Product GelrinC
Patent protects ready to use liquid formulation of GerlinC which improves efficiency and focus for surgeons, resulting in a 10-minute procedure
Robust IP portfolio includes 35 issued patents to date worldwide
GerlinC is in a pivotal FDA trial, has received regulatory approval in the EU, and is the world's first off-the-shelf regenerative treatment for knee cartilage repair
HERZLIYA, IL / ACCESS Newswire / December 18, 2025 / Regentis Biomaterials Ltd., ("Regentis" or the "Company") (NYSE American:RGNT), a regenerative medicine company focused on innovative tissue repair solutions, today announced that the U.S. Patent and Trademark Office has issued the Company a patent titled "Organic Solvent Free Compositions Compromising Protein-Polymer Conjugates and Uses Thereof" for its lead product, GerlinC®, addressing the liquid, ready to use version of the product, as well as improved processes for its production that avoid the use of organic solvents.
This patent's primary claims are focused around GelrinC's liquid ready-to-use formulation, reflecting Regentis' focus on simplifying procedures for surgeons while improving patient experience. GelrinC is delivered in liquid form to conform precisely to the geometry of the cartilage wound and is then cured using UV light to form an elastomeric, temporary, programmed implant. The straightforward, approximately 10-minute procedure eliminates complex preparation steps and distractions for surgeons and enables a brief, convenient office visit for patients.
"This most recent patent, our ninth in the U.S., comes at an important time as we recently surpassed 50% enrollment in our pivotal U.S. Food and Drug Administration ("FDA") trial for GelrinC in the treatment of knee cartilage repair," stated Regentis' Executive Chairman, Dr. Ehud Geller. "The patent provides broad protection for GelrinC through 2038, with the potential for additional extensions tied to future innovations. Our IP strategy pairs continuous patenting of new innovations with broadly drafted claims that strengthen and extend protection around our platform's core technology."
About GelrinC
Regentis' lead product, GelrinC®, is a cell-free, off-the-shelf hydrogel synchronized erosion and resorbable implant for the treatment of painful injuries to focal articular knee cartilage. As an innovative regenerative medical product, GelrinC offers an unprecedented solution that gives surgeons and payers an off-the-shelf, ready to use, simple to perform, reliable, and cost-effective procedure that provides patients with a single, 10-minute procedure, faster recovery, sustained pain relief, and functional improvement for more than 4 years, based on clinical study results to date. No effective off-the-shelf, ready to use treatment for focal knee cartilage defects is currently available on the market. GelrinC has CE Mark approval in the European Union and is now being evaluated in a pivotal U.S. Food and Drug Administration (FDA) study, which has completed over 50% enrollment.
About Regentis Biomaterials
Regentis Biomaterials Ltd is a regenerative medicine company dedicated to developing innovative tissue repair solutions that restore health and enhance quality of life. With an initial focus on orthopedic treatments, Regentis' Gelrin platform technology, based on synchronized, degradable hydrogel implants, regenerates damaged or diseased tissue including inflamed cartilage and bone. Regentis' lead product GelrinC, is a cell-free, off-the-shelf hydrogel that is eroded and resorbed in the knee, allowing the surrounding cells to regenerate the cartilage in a controlled and synchronous process. GelrinC aims to address a market of approximately 470,000 cases for cartilage knee repair annually in the U.S. where no off-the-shelf treatment is available.
Forward Looking Statements
This press release contains "forward-looking statements" that are subject to substantial risks and uncertainties. All statements, other than statements of historical fact, contained in this press release are forward-looking statements. Forward-looking statements contained in this press release may be identified by the use of words such as "anticipate," "believe," "contemplate," "could," "estimate," "expect," "intend," "seek," "may," "might," "plan," "potential," "predict," "project," "target," "aim," "should," "will" "would," or the negative of these words or other similar expressions, although not all forward-looking statements contain these words, and include the potential for extending Regentis' patent estate. Forward-looking statements are based on Regentis' current expectations and are subject to inherent uncertainties, risks and assumptions that are difficult to predict. Further, certain forward-looking statements are based on assumptions as to future events that may not prove to be accurate. Factors that may affect future results and may cause these forward-looking statements to be inaccurate include, without limitation: the ability of our clinical trials to demonstrate safety and efficacy of GelrinC or any future product candidate, and other positive results; the timing and focus of our preclinical studies and clinical trials, and the reporting of data from those studies and trials; the size of the market opportunity for of GelrinC or any future product candidate, including our estimates of the number of patients who suffer from the diseases we are targeting; our ability to accurately identify demand for product candidates; the success of competing therapies that are or may become available; the beneficial characteristics, safety, efficacy and therapeutic effects of our product candidates; our ability to obtain FDA approval for of GelrinC or any future product candidate and obtain and maintain regulatory approval; our ability to obtain market acceptance of of GelrinC or any future product candidate from the medical community and third-party payors; our plans relating to the further development of GelrinC or any future product candidate, including additional disease states or indications we may pursue; existing regulations and regulatory developments in the United States and other jurisdictions; our plans and ability to obtain or protect intellectual property rights, including extensions of patent terms where available and our ability to avoid infringing the intellectual property rights of others; the need to hire additional personnel and our ability to attract and retain such personnel; our estimates regarding expenses, future revenue, capital requirements and needs for additional financing; our dependence on third parties; our financial performance and our ability to repay our loans and debts; and our ability to negotiate favorable terms in any collaboration, licensing or other arrangements into which we may enter and perform our obligations under such collaborations. For a more detailed description of the risks and uncertainties affecting Regentis, reference is made to the Company's reports filed from time to time with the Securities and Exchange Commission ("SEC"), including, but not limited to, the risks detailed in the section titled "Risk Factors" in the final prospectus related to the public offering filed with the SEC. Forward-looking statements contained in this announcement are made as of this date, and Regentis undertakes no duty to update such information except as required under applicable law.
Contact:
SOURCE: Regentis Biomaterials Ltd
View the original press release on ACCESS Newswire
A.Anderson--AT



