-
 Thauvin on target again as Lens remain top in France
Thauvin on target again as Lens remain top in France
-
Greyness and solitude: French ex-president describes prison stay

-
 Frank relieved after Spurs ease pressure on under-fire boss
Frank relieved after Spurs ease pressure on under-fire boss
-
England kick off World Cup bid in Dallas as 2026 schedule confirmed
-
 Milei welcomes Argentina's first F-16 fighter jets
Milei welcomes Argentina's first F-16 fighter jets
-
No breakthrough at 'constructive' Ukraine-US talks

-
 Bielle-Biarrey double helps Bordeaux-Begles open Champions Cup defence with Bulls win
Bielle-Biarrey double helps Bordeaux-Begles open Champions Cup defence with Bulls win
-
Verstappen looking for a slice of luck to claim fifth title

-
 Kane cameo hat-trick as Bayern blast past Stuttgart
Kane cameo hat-trick as Bayern blast past Stuttgart
-
King Kohli says 'free in mind' after stellar ODI show

-
 Arsenal rocked by Aston Villa, Man City cut gap to two points
Arsenal rocked by Aston Villa, Man City cut gap to two points
-
Crestfallen Hamilton hits new low with Q1 exit

-
 Sleepless in Abu Dhabi - nervy times for Norris says Rosberg
Sleepless in Abu Dhabi - nervy times for Norris says Rosberg
-
Arsenal will bounce back from Villa blow: Arteta

-
 UN Security Council delegation urges all sides to stick to Lebanon truce
UN Security Council delegation urges all sides to stick to Lebanon truce
-
Verstappen outguns McLarens to take key pole in Abu Dhabi

-
 Syria's Kurds hail 'positive impact' of Turkey peace talks
Syria's Kurds hail 'positive impact' of Turkey peace talks
-
Verstappen takes pole position for season-ending Abu Dhabi GP

-
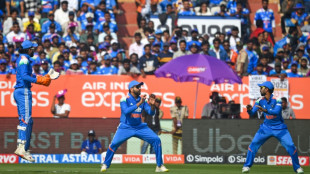 Jaiswal hits ton as India thrash S. Africa to clinch ODI series
Jaiswal hits ton as India thrash S. Africa to clinch ODI series
-
UK's Farage rallies in Scottish town hit by immigration protests

-
 Saracens kick off European campaign by crushing Clermont
Saracens kick off European campaign by crushing Clermont
-
Arsenal rocked by Villa as Buendia ends leaders' unbeaten run

-
 Venezuela's Machado vows to make Nobel Peace Prize ceremony
Venezuela's Machado vows to make Nobel Peace Prize ceremony
-
Kidnapping fears strain family bonds in Nigeria

-
 'Chosen' Mbappe on way to making Real Madrid history like Ronaldo: Alonso
'Chosen' Mbappe on way to making Real Madrid history like Ronaldo: Alonso
-
Russian strikes on Ukraine trigger heating, water cuts

-
 Mediators Qatar, Egypt call for next steps in Gaza truce
Mediators Qatar, Egypt call for next steps in Gaza truce
-
Olympic favourite Malinin pulls off stunning GP Final win

-
 Venezuela's Machado to receive peace prize in Oslo: Nobel Institute
Venezuela's Machado to receive peace prize in Oslo: Nobel Institute
-
Russell tops practice times to outpace title-chasing trio

-
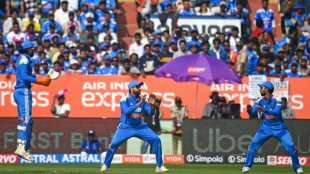 India bowl out South Africa for 270 after De Kock ton
India bowl out South Africa for 270 after De Kock ton
-
England staring down the barrel under Gabba lights as Australia dominate

-
 Egyptian actor faces challenge in iconic role of singer Umm Kulthum
Egyptian actor faces challenge in iconic role of singer Umm Kulthum
-
Chock and Bates win Grand Prix Final ice dance

-
 Starvation fears as flood toll passes 900 in Indonesia
Starvation fears as flood toll passes 900 in Indonesia
-
Four civilians, soldier killed in Afghan-Pakistan border clash

-
 Milan-Cortina chief admits venue time pinch as Olympic torch relay begins
Milan-Cortina chief admits venue time pinch as Olympic torch relay begins
-
England make quick start after Australia take big lead at Gabba

-
 Finally! India break toss jinx as Rahul gets lucky
Finally! India break toss jinx as Rahul gets lucky
-
Will EU give ground on 2035 combustion-engine ban?

-
 England nemesis Starc stretches Australia lead in Gabba Ashes Test
England nemesis Starc stretches Australia lead in Gabba Ashes Test
-
Banana skin 'double whammy' derails McIlroy at Australian Open

-
 Epic Greaves double ton earns West Indies draw in first NZ Test
Epic Greaves double ton earns West Indies draw in first NZ Test
-
Thunder roll to 14th straight NBA win, Celtics beat depleted Lakers

-
 Myanmar citizens head to early polls in Bangkok
Myanmar citizens head to early polls in Bangkok
-
Starvation fears as more heavy rain threaten flood-ruined Indonesia

-
 Sri Lanka unveils cyclone aid plan as rains persist
Sri Lanka unveils cyclone aid plan as rains persist
-
Avatar 3 aims to become end-of-year blockbuster

-
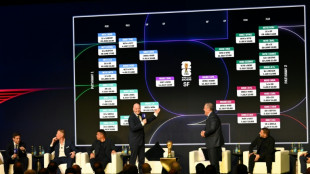
 Contenders plot path to 2026 World Cup glory after Trump steals show at draw
Contenders plot path to 2026 World Cup glory after Trump steals show at draw
-
Greaves leads dramatic West Indies run chase in NZ Test nail-biter

| RBGPF | 0% | 78.35 | $ | |
| GSK | -0.33% | 48.41 | $ | |
| RELX | -0.55% | 40.32 | $ | |
| SCS | -0.56% | 16.14 | $ | |
| AZN | 0.17% | 90.18 | $ | |
| NGG | -0.66% | 75.41 | $ | |
| BP | -3.91% | 35.83 | $ | |
| BTI | -1.81% | 57.01 | $ | |
| RYCEF | -0.34% | 14.62 | $ | |
| CMSC | -0.21% | 23.43 | $ | |
| BCC | -1.66% | 73.05 | $ | |
| VOD | -1.31% | 12.47 | $ | |
| RIO | -0.92% | 73.06 | $ | |
| BCE | 1.4% | 23.55 | $ | |
| JRI | 0.29% | 13.79 | $ | |
| CMSD | -0.3% | 23.25 | $ |

BrYet US, Inc. Receives Approval to Initiate in Australia a First-in-Human Phase 1/2 Trial of ML-016 in Advanced Cancers with Lung and/or Liver Involvement
HOUSTON, TX / ACCESS Newswire / October 6, 2025 / HOUSTON, TX and SUNSHINE COAST, QUEENSLAND / ACCESS Newswire / October 6, 2025 / BrYet US, Inc. ("BrYet"), a biotechnology innovator developing a drug with the potential to be a first-in-class medicine for metastatic disease, today announced that it has received an Australian Human Research Ethics Committee (HREC) approval for its first-in-human clinical study of ML-016.
The open-label, dose-escalation and expansion study, titled "A Phase 1/2 Study of ML-016 in Participants with Advanced Cancer with Lung and/or Liver Involvement," is authorized to commence in Australia with the University of the Sunshine Coast (UniSC) Clinical Trials as the lead site. BrYet expects the trial enrollment to begin in early 2026. The drug, ML-016 is manufactured and formulated at NerPharMa in Nerviano, Italy.
"Securing HREC approval is a critical milestone that brings us closer to providing hope of improved health to patients suffering from cancers of the lungs and liver, including metastatic lesions originating from other organs or tissue in the body," said Dr. Mauro Ferrari, the President and CEO of BrYet. "Furthermore, it supports our innovative approach to oncology, based upon targeting the molecular transport phenotype of cancer lesions in the lungs and liver."
ML-016 comprises an amino acid polymer conjugated to doxorubicin, with a formulation that includes platelet-like bio-erodible mesoporous silicon elements, which target the vascular endothelium of blood vessels in the tumor microenvironment. The amino acid-drug conjugate is released into the tumor, where it forms exosome-like vesicles. These "exosomoids" are designed to be preferentially taken up by cancer cells including those that have previously been resistant to therapy.
Through the mechanisms of cellular trafficking, the exosomoid vesicles are intended to be transported to late-stage endosomes and lysosomes, where the increased acidity releases the cytotoxic agent into the cell nucleus. In preclinical studies, BrYet demonstrated that by way of these combined mechanisms of action and transport, 50% of laboratory animals with otherwise uniformly lethal metastatic burden achieved long-term survival.
The Phase 1 portion of the trial will evaluate the safety, tolerability, and pharmacokinetics of ML-016 and identify two doses recommended for expansion into Phase 2. The Phase 2 expansion portion of the trial will include disease specific cohorts planned to explore preliminary antitumor activity in advanced solid tumors involving the lungs and/or liver. The trial will be led by UniSC Principal Investigator Dr. Brenton Seidl who believes metastatic cancer involving the lungs or liver remains one of the most complex challenges in oncology.
"This trial offers hope for those who have exhausted standard treatment options and allows us to investigate a potential new approach that may improve outcomes for patients with advanced solid cancers," Dr Seidl said.
###
About BrYet US, Inc.
BrYet is a privately held biotechnology company developing potentially first-in-class therapies for patients suffering from cancers for which there is no current curative treatment. BrYet's lead asset, ML-016, is being developed for cancers of the lungs and liver, including advanced primary malignancies and metastatic spread from primary cancer that originates in other organs or tissue of the body. The company's fundamental belief is that upon localization in the lungs and liver, these cancers acquire molecular transport phenotypes that are conserved regardless of site of origin and are largely independent of molecular mutations and their continued evolution. BrYet designs multi-component new chemical entities and formulations, which are directed against the fundamental aspects of these cancer-associated, organ-specific transport phenotypes. The company's proprietary platforms include the mesoporous silicon components, and the mathematical formalism for designing the multi-component drugs, termed Transport Oncophysics. BrYet believes that similar approaches may provide advances against other forms of presently incurable cancers, as well as other pathologies of the lungs and liver. For more information about BrYet, please visit the company's website: https://bryetpharma.com/
About UniSC Clinical Trials
UniSC Clinical Trials operates a growing clinical trial site network across the Sunshine Coast and surrounding regions, including specialist oncology trial capabilities. UniSC Clinical Trials works with local and global medical experts and industry to bring advanced treatments and therapies to life, and make cutting-edge medical research accessible to everyone. For more information, visit https://www.usc.edu.au/community/unisc-clinical-trials-clinical-trial-site-network
Safe-Harbor Statement
This press release contains forward-looking statements concerning BrYet and its business. These statements are based on the beliefs of, assumptions made by, and information currently available to the company's management. When used in this document, the words "expects," "anticipates," "estimates," "intends," "believes," "plans," "predicts," "should," "could," "will," and similar expressions are intended to identify forward-looking statements.
Forward-looking statements are subject to risks and uncertainties that could cause actual results to differ from expectations. Factors that could contribute to these differences include the results of studies and clinical trials, regulatory approvals, challenges in clinical trials, the ability to retain employees, research and development expenses, reliance on third parties, intellectual property issues, competition, future funding needs, economic conditions, and other industry-specific risks. You should not place undue reliance on these statements, which are current as of the date of this press release. BrYet does not plan to update these statements unless legally required.
Media & Investor Contact
BrYet US, Inc.
[email protected] | bryetpharma.com
2450 Holcombe Blvd., Suite 1520, Houston, Texas 77021, USA
SOURCE: BrYet US Inc.
View the original press release on ACCESS Newswire
A.Taylor--AT