-
 Stocks diverge as rate hopes rise, AI fears ease
Stocks diverge as rate hopes rise, AI fears ease
-
Man City players face Christmas weigh-in as Guardiola issues 'fatty' warning

-
 German Christmas markets hit by flood of fake news
German Christmas markets hit by flood of fake news
-
Liverpool fear Isak has broken leg: reports

-
 West Indies captain says he 'let the team down' in New Zealand Tests
West Indies captain says he 'let the team down' in New Zealand Tests
-
Thailand says Cambodia agrees to border talks after ASEAN meet

-
 Alleged Bondi shooters conducted 'tactical' training in countryside, Australian police say
Alleged Bondi shooters conducted 'tactical' training in countryside, Australian police say
-
Swiss court to hear landmark climate case against cement giant

-
 Steelers beat Lions in 'chaos' as three NFL teams book playoffs
Steelers beat Lions in 'chaos' as three NFL teams book playoffs
-
Knicks' Brunson scores 47, Bulls edge Hawks epic

-
 Global nuclear arms control under pressure in 2026
Global nuclear arms control under pressure in 2026
-
Five-wicket Duffy prompts West Indies collapse as NZ win series 2-0

-
 Asian markets rally with Wall St as rate hopes rise, AI fears ease
Asian markets rally with Wall St as rate hopes rise, AI fears ease
-
Jailed Malaysian ex-PM Najib loses bid for house arrest

-
 Banned film exposes Hong Kong's censorship trend, director says
Banned film exposes Hong Kong's censorship trend, director says
-
Duffy, Patel force West Indies collapse as NZ close in on Test series win

-
 Australian state pushes tough gun laws, 'terror symbols' ban after shooting
Australian state pushes tough gun laws, 'terror symbols' ban after shooting
-
A night out on the town during Nigeria's 'Detty December'
-
 US in 'pursuit' of third oil tanker in Caribbean: official
US in 'pursuit' of third oil tanker in Caribbean: official
-
CO2 soon to be buried under North Sea oil platform

-
 Steelers edge Lions as Bears, 49ers reach playoffs
Steelers edge Lions as Bears, 49ers reach playoffs
-
India's Bollywood counts costs as star fees squeeze profits

-
 McCullum admits errors in Ashes preparations as England look to salvage pride
McCullum admits errors in Ashes preparations as England look to salvage pride
-
Pets, pedis and peppermints: When the diva is a donkey

-
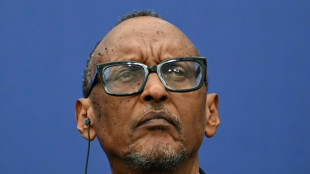 'A den of bandits': Rwanda closes thousands of evangelical churches
'A den of bandits': Rwanda closes thousands of evangelical churches
-
Southeast Asia bloc meets to press Thailand, Cambodia on truce

-
 As US battles China on AI, some companies choose Chinese
As US battles China on AI, some companies choose Chinese
-
AI resurrections of dead celebrities amuse and rankle

-
 EON Resources Inc. Reports Management and Directors Buy an Additional 282,000 Shares of EON Class A Common Stock for a Total of 1,561,000 Shares Bought in 2025 and a Total Ownership of Over 5 million Shares
EON Resources Inc. Reports Management and Directors Buy an Additional 282,000 Shares of EON Class A Common Stock for a Total of 1,561,000 Shares Bought in 2025 and a Total Ownership of Over 5 million Shares
-
Heirs Energies Agrees $750m Afreximbank Financing to Drive Long-Term Growth

-
 Black Book Poll: "Governed AI" Emerges as the Deciding Factor in 2026 NHS Procurement
Black Book Poll: "Governed AI" Emerges as the Deciding Factor in 2026 NHS Procurement
-
Hemogenyx Pharmaceuticals PLC Announces Update on Admission of Shares

-
 Pantheon Resources PLC Announces Shareholder Letter and Corporate Update on Dubhe-1
Pantheon Resources PLC Announces Shareholder Letter and Corporate Update on Dubhe-1
-
Tocvan Begins Trenching Material for the Pilot Mine and Pushes Ahead With Infrastructure Development

-
 Steelers receiver Metcalf strikes Lions fan
Steelers receiver Metcalf strikes Lions fan
-
Morocco coach 'taking no risks' with Hakimi fitness

-
 Gang members given hundreds-years-long sentences in El Salvador
Gang members given hundreds-years-long sentences in El Salvador
-
Chargers, Bills edge closer to playoff berths

-
 US, Ukraine hail 'productive' Miami talks but no breakthrough
US, Ukraine hail 'productive' Miami talks but no breakthrough
-
Gang members given hundred-years-long sentences in El Salvador

-
 Hosts Morocco off to winning start at Africa Cup of Nations
Hosts Morocco off to winning start at Africa Cup of Nations
-
No jacket required for Emery as Villa dream of title glory

-
 Amorim fears United captain Fernandes will be out 'a while'
Amorim fears United captain Fernandes will be out 'a while'
-
Nigerian government frees 130 kidnapped Catholic schoolchildren

-
 Captain Kane helps undermanned Bayern go nine clear in Bundesliga
Captain Kane helps undermanned Bayern go nine clear in Bundesliga
-
Trump administration denies cover-up over redacted Epstein files

-
 Captain Kane helps undermanned Bayern go nine clear
Captain Kane helps undermanned Bayern go nine clear
-
Rogers stars as Villa beat Man Utd to boost title bid

-
 Barca strengthen Liga lead at Villarreal, Atletico go third
Barca strengthen Liga lead at Villarreal, Atletico go third
-
Third 'Avatar' film soars to top in N. American box office debut


Positive Interim 2-Year Data from enVVeno Medical's VenoValve Pivotal Study to be Presented Today at the Society for Vascular Surgery (SVS) 2025 Vascular Annual Meeting
Interim two-year follow-up data from 42 subjects in the VenoValve pivotal trial show sustained clinical improvement and continued patient benefit at 24 months compared to baseline
83% of subjects maintained a clinically meaningful benefit with a 3 or more point improvement in Revised Venous Clinical Severity Score (rVCSS)
9.1 point average rVCSS improvement among the clinically meaningful benefit cohort
Subjects experienced a median 74% improvement in leg pain
Interim follow-up data indicate sustained improvements across all venous-specific quality-of-life (QoL) indicators
A decision from the U.S. Food and Drug Administration (FDA) on the VenoValve is anticipated in the second half of 2025
Company to host live webcast with presenting Principal Investigator, today, June 6th at 11:20 AM ET / 10:20 AM CT - Access the Webcast Here
IRVINE, CA / ACCESS Newswire / June 6, 2025 / enVVeno Medical Corporation (Nasdaq:NVNO) ("enVVeno" or the "Company"), a company setting new standards of care for the treatment of deep venous disease, today announced that interim two-year follow-up data on 42 subjects from the 75 person VenoValve U.S. pivotal trial will be presented today by lead enroller Dr. Cassius Iyad Ochoa Chaar, at the Society for Vascular Surgery (SVS) 2025 Vascular Annual Meeting (VAM25) being held June 4-7, 2025 in New Orleans, LA. Additionally, the Company announced it will host a live webcast to discuss these interim results today, Friday, June 6th at 11:20 AM ET / 10:20 AM CT (details below).
Key interim two-year follow-up data being presented at VAM25 include:
83.3% of subjects (n=35/42) maintained a clinically meaningful benefit, defined as an improvement of 3 or more points in the revised Venous Clinical Severity Score (rVCSS).
9.1 point average rVCSS improvement among the responder cohort.
A median 74% improvement in leg pain, as measured by the Visual Analog Scale (VAS).
Wound healing outcomes in 17 subjects with 25 ulcers showed that 60% of ulcers healed completely, 24% decreased in size, and 16% increased in size.
Patient-reported outcomes also demonstrated sustained improvements across all venous specific QoL indicators (VEINES-QoL/Sym).
Among the subjects (n=30), a 100% valve patency rate.
All values were calculated comparing each patient's baseline levels to the reported values at the patient's 24-month visit. The Revised Venous Clinical Severity Score (rVCSS) is a clinically validated scoring system used to track the progression or regression of venous diseases.
"These interim two-year follow-up data demonstrate substantial and sustained improvement across all effectiveness endpoints at two years. This is extremely encouraging, especially when you consider that all the patients enrolled in the study had severe CVI and failed all other treatment options," said Robert Berman, enVVeno Medical's Chief Executive Officer. "Despite attempts over many decades, nobody has been able to create an effective treatment for severe CVI caused by malfunctioning valves in the deep veins of the leg. The VenoValve has the potential to change the treatment paradigm for deep venous CVI, both for the millions of patients suffering from severe CVI and the thousands of vascular surgeons who have been waiting for an effective treatment option."
Dr. Cassius Iyad Ochoa Chaar, who is the Associate Professor of Surgery, Division of Vascular Surgery and Endovascular Therapy, Yale School of Medicine, will present the abstract titled, "Patients with Deep Venous Reflux Continue to Experience Clinical Improvement 2-year after Implantation of the VenoValve" today at VAM 2025.
The VenoValve is a potential first-in-class, surgical replacement venous valve for patients with severe deep venous CVI. The Company estimates that there are approximately 2.5 million potential new patients each year in the U.S. that could be candidates for the VenoValve. The Company has submitted a pre-market authorization (PMA) application for the VenoValve to the U.S. Food and Drug Administration (FDA), with a decision anticipated in the second half of 2025.
Webcast Details
The Company will host a webcast presentation to discuss the results for investors, analysts and other interested parties today, June 6, 2025, at 11:20 AM ET / 10:20 AM CT. Joining enVVeno management for the event will be Dr. Chaar. The live webcast will be accessible on the Events page of the enVVeno website, envveno.com, and will be archived for 90 days.
About CVI
Severe, deep venous Chronic Venous Insufficiency (CVI) is a debilitating disease that is most often caused by blood clots (deep vein thromboses or DVTs) in the deep veins of the leg. When valves inside of the veins of the leg fail, blood flows in the wrong direction and pools in the lower leg, causing pressure within the veins of the leg to increase (venous hypertension). Symptoms of severe CVI include leg swelling, pain, edema, and in the most severe cases, recurrent open sores known as venous ulcers. The disease can severely impact everyday functions such as sleeping, bathing, dressing, and walking, and is known to result in high rates of depression and anxiety. There are currently no effective treatments for severe CVI of the deep vein system caused by valvular incompetence. Estimates indicate that CVI costs the U.S. healthcare system in excess of $4 billion each year.
About enVVeno Medical Corporation
enVVeno Medical (NASDAQ:NVNO) is an Irvine, California-based, late clinical-stage medical device Company focused on the advancement of innovative bioprosthetic (tissue-based) solutions to improve the standard of care for the treatment of deep venous disease. The Company's lead product, the VenoValve®, is a first-in-class surgical replacement venous valve being developed for the treatment of deep venous Chronic Venous Insufficiency (CVI). The Company is also developing a non-surgical, transcatheter based replacement venous valve for the treatment of deep venous CVI called enVVe®. Both the VenoValve and enVVe are designed to act as one-way valves, to help assist in propelling blood up the leg, and back to the heart and lungs. The VenoValve is currently being evaluated in the VenoValve U.S. pivotal study and the Company is currently performing the final testing necessary to seek approval for the pivotal trial for enVVe.
Cautionary Note on Forward-Looking Statements
This press release and any statements of stockholders, directors, employees, representatives and partners of enVVeno Medical Corporation (the "Company") related thereto contain, or may contain, among other things, certain "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements involve significant risks and uncertainties. Such statements may include, without limitation, statements identified by words such as "projects," "may," "will," "could," "would," "should," "believes," "expects," "anticipates," "estimates," "intends," "plans," "potential" or similar expressions. These statements are based upon the current beliefs and expectations of the Company's management and are subject to significant risks and uncertainties, including those detailed in the Company's filings with the Securities and Exchange Commission. Actual results and timing (may differ significantly from those set forth or implied in the forward-looking statements. Forward-looking statements involve certain risks and uncertainties that are subject to change based on various factors (many of which are beyond the Company's control). The Company undertakes no obligation to publicly update any forward-looking statements, whether as a result of new information, future presentations or otherwise, except as required by applicable law.
INVESTOR CONTACT:
Jenene Thomas, JTC Team, LLC
[email protected]
(908) 824-0775
MEDIA CONTACT:
Glenn Silver, FINN Partners
[email protected]
(973) 818-8198
SOURCE: enVVeno Medical Corporation
View the original press release on ACCESS Newswire
M.King--AT