-
 Knicks' Brunson scores 47, Bulls edge Hawks epic
Knicks' Brunson scores 47, Bulls edge Hawks epic
-
Global nuclear arms control under pressure in 2026

-
 Five-wicket Duffy prompts West Indies collapse as NZ win series 2-0
Five-wicket Duffy prompts West Indies collapse as NZ win series 2-0
-
Asian markets rally with Wall St as rate hopes rise, AI fears ease

-
 Jailed Malaysian ex-PM Najib loses bid for house arrest
Jailed Malaysian ex-PM Najib loses bid for house arrest
-
Banned film exposes Hong Kong's censorship trend, director says

-
 Duffy, Patel force West Indies collapse as NZ close in on Test series win
Duffy, Patel force West Indies collapse as NZ close in on Test series win
-
Australian state pushes tough gun laws, 'terror symbols' ban after shooting

-
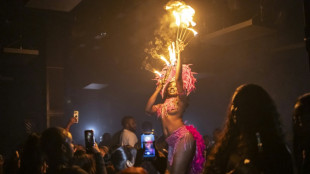 A night out on the town during Nigeria's 'Detty December'
A night out on the town during Nigeria's 'Detty December'
-
US in 'pursuit' of third oil tanker in Caribbean: official

-
 CO2 soon to be buried under North Sea oil platform
CO2 soon to be buried under North Sea oil platform
-
Steelers edge Lions as Bears, 49ers reach playoffs

-
 India's Bollywood counts costs as star fees squeeze profits
India's Bollywood counts costs as star fees squeeze profits
-
McCullum admits errors in Ashes preparations as England look to salvage pride

-
 Pets, pedis and peppermints: When the diva is a donkey
Pets, pedis and peppermints: When the diva is a donkey
-
'A den of bandits': Rwanda closes thousands of evangelical churches
-
 Southeast Asia bloc meets to press Thailand, Cambodia on truce
Southeast Asia bloc meets to press Thailand, Cambodia on truce
-
As US battles China on AI, some companies choose Chinese

-
 AI resurrections of dead celebrities amuse and rankle
AI resurrections of dead celebrities amuse and rankle
-
Steelers receiver Metcalf strikes Lions fan

-
 Morocco coach 'taking no risks' with Hakimi fitness
Morocco coach 'taking no risks' with Hakimi fitness
-
Gang members given hundreds-years-long sentences in El Salvador

-
 Chargers, Bills edge closer to playoff berths
Chargers, Bills edge closer to playoff berths
-
US, Ukraine hail 'productive' Miami talks but no breakthrough

-
 Gang members given hundred-years-long sentences in El Salvador
Gang members given hundred-years-long sentences in El Salvador
-
Hosts Morocco off to winning start at Africa Cup of Nations

-
 No jacket required for Emery as Villa dream of title glory
No jacket required for Emery as Villa dream of title glory
-
Amorim fears United captain Fernandes will be out 'a while'

-
 Nigerian government frees 130 kidnapped Catholic schoolchildren
Nigerian government frees 130 kidnapped Catholic schoolchildren
-
Captain Kane helps undermanned Bayern go nine clear in Bundesliga

-
 Trump administration denies cover-up over redacted Epstein files
Trump administration denies cover-up over redacted Epstein files
-
Captain Kane helps undermanned Bayern go nine clear

-
 Rogers stars as Villa beat Man Utd to boost title bid
Rogers stars as Villa beat Man Utd to boost title bid
-
Barca strengthen Liga lead at Villarreal, Atletico go third

-
 Third 'Avatar' film soars to top in N. American box office debut
Third 'Avatar' film soars to top in N. American box office debut
-
Third day of Ukraine settlement talks to begin in Miami

-
 Barcelona's Raphinha, Yamal strike in Villarreal win
Barcelona's Raphinha, Yamal strike in Villarreal win
-
Macron, on UAE visit, announces new French aircraft carrier

-
 Barca's Raphinha, Yamal strike in Villarreal win
Barca's Raphinha, Yamal strike in Villarreal win
-
Gunmen kill 9, wound 10 in South Africa bar attack

-
 Allegations of new cover-up over Epstein files
Allegations of new cover-up over Epstein files
-
Atletico go third with comfortable win at Girona

-
 Schwarz breaks World Cup duck with Alta Badia giant slalom victory
Schwarz breaks World Cup duck with Alta Badia giant slalom victory
-
Salah unaffected by Liverpool turmoil ahead of AFCON opener - Egypt coach

-
 Goggia eases her pain with World Cup super-G win as Vonn takes third
Goggia eases her pain with World Cup super-G win as Vonn takes third
-
Goggia wins World Cup super-G as Vonn takes third

-
 Cambodia says Thai border clashes displace over half a million
Cambodia says Thai border clashes displace over half a million
-
Kremlin denies three-way US-Ukraine-Russia talks in preparation

-
 Williamson says 'series by series' call on New Zealand Test future
Williamson says 'series by series' call on New Zealand Test future
-
Taiwan police rule out 'terrorism' in metro stabbing


Petros Pharmaceuticals Partners with Innolitics, a Leading Software-as-a-Medical-Device Developer
With more than $10m in cash, and a significantly reduced burn rate, the Company is positioned to fully fund the development of its technology platform.
NEW YORK, NY / ACCESS Newswire / May 20, 2025 / Petros Pharmaceuticals, Inc. (NASDAQ:PTPI) ("Petros" or the "Company"), a company focused on expanding consumer access to medication through over the counter ("OTC") drug development programs, today announces its partnership with Innolitics, a leading developer in the Software-as-a-Medical Device space. The collaboration is intended to integrate many of the Company's emerging capabilities in the ongoing development of its first-in-industry SaaS platform designed to complement its proprietary web application (also known as a Software as a Medical Device, or "SaMD"). Utilizing the same knowledge management system as multiple Fortune 100 companies and having developed software for more than 70 medical devices, Innolitics is expected to be integrating many of the Company's recently announced cloud-based components. These enhancements include AI, cybersecurity, and others to provide potential prescription treatments and pharmaceutical sponsors with a commercially viable and enterprise scaled platform.
Innolitics specializes in medical device software development, cybersecurity, and FDA regulatory submissions. It is expected to be integral to integrations with the Company's Big Data partner and consumer user interface experience. Their team comprises a multi-faceted array of talent including software engineers, cybersecurity experts, regulatory consultants, and AI and machine learning processing experts.
Fady Boctor, Petros' President and Chief Commercial Officer, commented, "We continue to make incremental progress in the development of our enterprise system designed to take advantage of what we believe represents a significant shift in the pharmaceutical industry. We believe that our $10 million cash position, coupled with a significantly reduced burn rate compared to Q4 2024, puts us in good stead to complete this development and create a much-needed resource for the pharmaceutical industry in an age where new alternatives to expand access are in demand.
"As manufacturers face critical lifecycle management decisions, and the federal government has mandated new and better solutions for access to medical care, we believe our SaaS program will be key to overcoming longstanding challenges such as retail integration, electronic health record interoperability and the commercial scalability necessary for a true over-the-counter experience. This collaboration with Innolitics represents continued progress in the development of this system, and we are confident in both our approach and its potential in the larger market," concluded Mr. Boctor.
Petros' enterprise platform is being designed within the United States Food and Drug Administration's (the "FDA") Additional Conditions for Nonprescription Use ("ACNU") guidelines to facilitate bringing prescription products over the counter, and is intended to help industry comply with President Trump's recent Executive Order to expand access to medicines through OTC approvals under his Make America Healthy Again (MAHA) initiative.
The Company is developing this intended licensable platform to provide a variety of services, including patient self-selection tools, electronic health records integration to support appropriate patient use, possible integration with retail pharmacies, and robust cybersecurity and privacy safeguards. The ACNU regulatory framework provides guidelines that are designed to expand access as pharmaceutical companies evaluate various potential switches from prescription to OTC availability.
Petros' system is being designed to meet these stipulations in an efficient, commercially viable, licensable single framework. The Company believes there are multiple indications that may be appropriate for an Rx-to-OTC switch, including erectile dysfunction, hypercholesterolemia (high cholesterol), migraine, anxiety and urinary tract infection.
The emerging self-care market is currently estimated to be valued at over $38 billion with an expected compounded annual growth rate of 5.6% over the next 10 years.1
Rx-to-OTC Switches Market Size, Trends & Forecast 2033 | FMI (futuremarketinsights.com)
About Petros Pharmaceuticals
Petros Pharmaceuticals, Inc. is committed to the goal of becoming a leading innovator in the emerging $38 billion self-care market by providing expanded access to key prescription pharmaceuticals as OTC treatment options. The Company is currently developing a proprietary SaaS platform and a proprietary SaMD web application designed to assist pharmaceutical companies in meeting FDA standards to assist in the Rx-to-OTC switch.
About the Pathway from Rx to OTC
The process of switching a prescription medication to OTC first involves the design of a Drug Facts Label ("DFL") that is well understood by potential consumers. Then, data must show that consumers can make an appropriate informed decision to use or not to use the product based only upon the information on the DFL and their personal medical history. Consumers must then demonstrate that they can properly use the product based upon the information on the DFL. To accomplish this, the FDA ordinarily requires a consumer tested OTC DFL. Such testing includes conduct of iterative Label Comprehension Studies (LCS) in the general population, Self-Selection Studies (SSS) in a population interested in using the product and in specific populations who may be harmed if they use the product, and usually one Actual Use Trial (AUT) demonstrating safe and appropriate use by consumers in a simulated OTC setting.
The regulation that the FDA recently finalized introduced Additional Conditions for Nonprescription Use ("ACNU") criteria that enable correct self-selection by consumers and may expand OTC access to medications that formerly could only be available by prescription. An ACNU may be an innovative computerized tool, or the additional conditions may use other approaches that support the switch process. Petros is developing a technology platform (SaaS) to assist companies in navigating this pathway.
Cautionary Note Regarding Forward-Looking Statements
This press release includes forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. These forward-looking statements are based upon the Company management's assumptions, expectations, projections, intentions, and beliefs about future events. In some cases, predictive, future-tense or forward-looking words such as "intend," "develop," "goal," "plan," "predict", "may," "will," "project," "estimate," "anticipate," "believe," "expect," "continue," "potential," "opportunity," "forecast," "should," "target," "strategy" and similar expressions, whether in the negative or affirmative, that reflect our current views with respect to future events and operational, economic and financial performance are intended to identify forward-looking statements, but are not the exclusive means of identifying such statements. Such forward-looking statements are only predictions, and actual results and the timing of certain events and circumstances may differ materially from those described by the forward-looking statements as a result of risks and uncertainties, Petros' ability to execute on its business strategy, including its plans to develop and commercialize its proprietary Rx-to-OTC switch technology Petros' ability to comply with obligations as a public reporting company; Petros' expectations related to the Company's partnership with Innolitics; Petros' ability to regain and maintain compliance with the Nasdaq Stock Market's listing standards; risks related to Petros' ability to continue as a going concern; risks related to Petros' history of incurring significant losses; and risks related to Petros' ability to obtain regulatory approvals for, or market acceptance of, any of its products or product candidates, including its proprietary Rx-to-OTC switch technology. Additional factors that could cause actual results to differ materially from the results anticipated in these forward-looking statements are contained in the Company's periodic reports and in other filings that the Company has filed, or may file, with the U.S. Securities and Exchange Commission (the "SEC") under the headings "Risk Factors" and "Management's Discussion and Analysis of Financial Condition and Results of Operations" and elsewhere. The Company cautions readers that the forward-looking statements included in this press release represent our beliefs, expectations, estimates and assumptions only as of the date of hereof and are not intended to give any assurance as to future results. New factors emerge from time to time, and it is not possible for us to predict all these factors. Further, the Company cannot assess the effect of each such factor on our business or the extent to which any factor, or combination of factors, may cause actual results to be materially different from those contained in any forward-looking statement. Accordingly, you should not unduly rely on any forward-looking statements.
The Company undertakes no obligation to update or revise any forward-looking statements contained in this press release, whether as a result of new information, future events, a change in our views or expectations or otherwise, except as required by federal securities laws.
Contacts
Investors:
CORE IR
[email protected]
Media:
Jules Abraham
CORE IR
917-885-7378
[email protected]
SOURCE: Petros Pharmaceuticals, Inc.
View the original press release on ACCESS Newswire
Ch.P.Lewis--AT