-
 Trump says Iran wants deal, US 'armada' larger than in Venezuela raid
Trump says Iran wants deal, US 'armada' larger than in Venezuela raid
-
US Justice Dept releases new batch of documents, images, videos from Epstein files

-
 Four memorable showdowns between Alcaraz and Djokovic
Four memorable showdowns between Alcaraz and Djokovic
-
Russian figure skating prodigy Valieva set for comeback -- but not at Olympics

-
 Barcelona midfielder Lopez agrees contract extension
Barcelona midfielder Lopez agrees contract extension
-
Djokovic says 'keep writing me off' after beating Sinner in late-nighter

-
 US Justice Dept releasing new batch of Epstein files
US Justice Dept releasing new batch of Epstein files
-
South Africa and Israel expel envoys in deepening feud

-
 French eyewear maker in spotlight after presidential showing
French eyewear maker in spotlight after presidential showing
-
Olympic dream 'not over', Vonn says after crash

-
 Brazil's Lula discharged after cataract surgery
Brazil's Lula discharged after cataract surgery
-
US Senate races to limit shutdown fallout as Trump-backed deal stalls

-
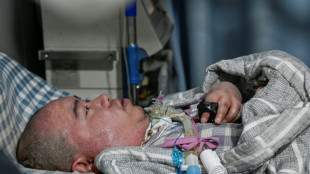
 'He probably would've survived': Iran targeting hospitals in crackdown
'He probably would've survived': Iran targeting hospitals in crackdown
-
Djokovic stuns Sinner to set up Australian Open final with Alcaraz

-
 Mateta omitted from Palace squad to face Forest
Mateta omitted from Palace squad to face Forest
-
Gold, silver prices tumble as investors soothed by Trump's Fed pick

-
 Trump attorney general orders arrest of ex-CNN anchor covering protests
Trump attorney general orders arrest of ex-CNN anchor covering protests
-
Djokovic 'pushed to the limit' in stunning late-night Sinner upset

-
 Tunisia's famed blue-and-white village threatened after record rains
Tunisia's famed blue-and-white village threatened after record rains
-
Top EU official voices 'shock' at Minneapolis violence

-
 Kremlin says agreed to halt strikes on Kyiv until Sunday
Kremlin says agreed to halt strikes on Kyiv until Sunday
-
Carrick calls for calm after flying start to Man Utd reign

-
 Djokovic to meet Alcaraz in Melbourne final after five-set marathon
Djokovic to meet Alcaraz in Melbourne final after five-set marathon
-
Italian officials to testify in trial over deadly migrant shipwreck

-
 Iran says defence capabilities 'never' up for negotiation
Iran says defence capabilities 'never' up for negotiation
-
UN appeals for more support for flood-hit Mozambicans

-
 Lijnders urges Man City to pile pressure on Arsenal in title race
Lijnders urges Man City to pile pressure on Arsenal in title race
-
Fulham sign Man City winger Oscar Bobb

-
 Strasbourg's Argentine striker Panichelli sets sights on PSG, World Cup
Strasbourg's Argentine striker Panichelli sets sights on PSG, World Cup
-
Jesus 'made love': Colombian president irks Christians with steamy claim

-
 IAEA board meets over Ukraine nuclear safety concerns
IAEA board meets over Ukraine nuclear safety concerns
-
Eurozone growth beats 2025 forecasts despite Trump woes

-
 Israel to partially reopen Gaza's Rafah crossing on Sunday
Israel to partially reopen Gaza's Rafah crossing on Sunday
-
Dutch PM-elect Jetten says not yet time to talk to Putin

-
 Social media fuels surge in UK men seeking testosterone jabs
Social media fuels surge in UK men seeking testosterone jabs
-
Forest face Fenerbahce, Celtic draw Stuttgart in Europa League play-offs
-
 US speed queen Vonn crashes at Crans-Montana, one week before Olympics
US speed queen Vonn crashes at Crans-Montana, one week before Olympics
-
Trump nominates former US Fed official as next central bank chief

-
 Alcaraz defends controversial timeout after beaten Zverev fumes
Alcaraz defends controversial timeout after beaten Zverev fumes
-
New Dutch government pledges ongoing Ukraine support

-
 Newcastle still coping with fallout from Isak exit, says Howe
Newcastle still coping with fallout from Isak exit, says Howe
-
Chad, France eye economic cooperation as they reset strained ties

-
 Real Madrid to play Benfica, PSG face Monaco in Champions League play-offs
Real Madrid to play Benfica, PSG face Monaco in Champions League play-offs
-
Everton winger Grealish set to miss rest of season in World Cup blow

-
 Trump brands Minneapolis nurse killed by federal agents an 'agitator'
Trump brands Minneapolis nurse killed by federal agents an 'agitator'
-
Arteta focuses on the positives despite Arsenal stumble

-
 Fijian Drua sign France international back Vakatawa
Fijian Drua sign France international back Vakatawa
-
Kevin Warsh, a former Fed 'hawk' now in tune with Trump

-
 Zverev rails at Alcaraz timeout in 'one of the best battles ever'
Zverev rails at Alcaraz timeout in 'one of the best battles ever'
-
Turkey leads Iran diplomatic push as Trump softens strike threat

| RBGPF | 1.65% | 83.78 | $ | |
| SCS | 0.12% | 16.14 | $ | |
| RYCEF | -2.69% | 16 | $ | |
| CMSC | 0.02% | 23.7 | $ | |
| BCC | -0.97% | 79.4 | $ | |
| BTI | -0.12% | 60.135 | $ | |
| RELX | -1.69% | 35.565 | $ | |
| NGG | -0.81% | 84.37 | $ | |
| GSK | 1.33% | 51.34 | $ | |
| RIO | -4.86% | 90.725 | $ | |
| JRI | 0.32% | 12.997 | $ | |
| CMSD | -0.12% | 24.03 | $ | |
| BCE | -0.18% | 25.44 | $ | |
| VOD | -0.58% | 14.625 | $ | |
| AZN | 0.75% | 93.285 | $ | |
| BP | -1.1% | 37.625 | $ |

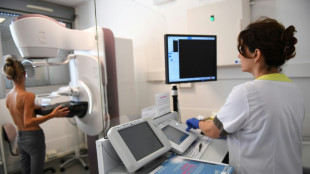
US health experts reassess hormone replacement therapy risks
US health authorities on Thursday began a reassessment of the risks surrounding Hormone Replacement Therapy (HRT), a treatment used by menopausal women around the world but long clouded by fear over its side effects.
HRT is taken to replace estrogen the body stops producing after menopause -- when periods end permanently -- and helps relieve symptoms such as hot flashes, vaginal discomfort, and pain during sex.
But its use has plummeted in recent years amid concerns including a potential link to invasive breast cancer.
Food and Drug Administration (FDA) chief Marty Makary, who convened Thursday's meeting of outside experts, has long advocated for HRT, saying its risks have been overstated.
"For decades, hormone replacement therapy for women -- that is estrogen or estrogen plus progesterone -- has helped women alleviate the symptoms of menopause, including hot flashes, dryness, mood swings, weight gain and poor sleep quality, to name a few," he said in a video ahead of the meeting.
He added that when initiated within a decade of the onset of the transitional period before menopause, HRT may even reduce cognitive decline, the risk of Alzheimer's, and prevent osteoporosis and cardiovascular disease.
Makary blamed the drop in HRT use on a landmark clinical trial, the Women's Health Initiative, which was halted in the early 2000s after it flagged increased risks of breast cancer and stroke. But he said subsequent studies had not replicated the findings on breast cancer.
"The many benefits of hormone therapy were ignored as it was seen as a carcinogen. Prescriptions for hormone replacement therapy plummeted in the United States, women flushed their pills down the toilet," he said Thursday.
"Fifty million plus women have not been offered the incredible potential health benefits of hormone replacement therapy because of medical dogma," he added, including his own mother, who suffered multiple bone fractures in her older life.
Critics of the trial argue it was flawed because the participants were too far from menopause, when risks are elevated and benefits limited, and that the formulations used are now outdated.
- Label changes -
Still, the issue remains divisive within the medical community.
The FDA's own warning label for HRT -- which can be administered through various means including orally, through skin patches, or vaginally -- cites risks including endometrial cancer, breast cancer, and life-threatening blood clots.
This week, the American Family Physician journal published an editorial that found limited benefits and significant harms associated with HRT.
"Menopause is a positive life experience for many women and should not be medicalized," the authors concluded.
The nature of the FDA expert meeting is also unusual. Unlike standard practice before the Trump administration, no agenda was publicly posted.
Several of the named panelists have ties to companies offering menopause treatments or who belong to the advocacy group "Let's Talk Menopause," which receives funding from pharmaceutical companies and campaigns to revise the FDA warning label.
R.Garcia--AT