-
 Ko, Woad share lead at LPGA season opener
Ko, Woad share lead at LPGA season opener
-
US Senate votes on funding deal - but shutdown still imminent

-
 US charges prominent journalist after Minneapolis protest coverage
US charges prominent journalist after Minneapolis protest coverage
-
Trump expects Iran to seek deal to avoid US strikes

-
 US Justice Dept releases documents, images, videos from Epstein files
US Justice Dept releases documents, images, videos from Epstein files
-
Guterres warns UN risks 'imminent financial collapse'

-
 NASA delays Moon mission over frigid weather
NASA delays Moon mission over frigid weather
-
First competitors settle into Milan's Olympic village

-
 Fela Kuti: first African to get Grammys Lifetime Achievement Award
Fela Kuti: first African to get Grammys Lifetime Achievement Award
-
Cubans queue for fuel as Trump issues oil ultimatum

-
 'Schitt's Creek' star Catherine O'Hara dead at 71
'Schitt's Creek' star Catherine O'Hara dead at 71
-
Curran hat-trick seals 11 run DLS win for England over Sri Lanka

-
 Cubans queue for fuel as Trump issues energy ultimatum
Cubans queue for fuel as Trump issues energy ultimatum
-
France rescues over 6,000 UK-bound Channel migrants in 2025

-
 Surprise appointment Riera named Frankfurt coach
Surprise appointment Riera named Frankfurt coach
-
Maersk to take over Panama Canal port operations from HK firm

-
 US arrests prominent journalist after Minneapolis protest coverage
US arrests prominent journalist after Minneapolis protest coverage
-
Analysts say Kevin Warsh a safe choice for US Fed chair

-
 Trump predicts Iran will seek deal to avoid US strikes
Trump predicts Iran will seek deal to avoid US strikes
-
US oil giants say it's early days on potential Venezuela boom

-
 Fela Kuti to be first African to get Grammys Lifetime Achievement Award
Fela Kuti to be first African to get Grammys Lifetime Achievement Award
-
Trump says Iran wants deal, US 'armada' larger than in Venezuela raid

-
 US Justice Dept releases new batch of documents, images, videos from Epstein files
US Justice Dept releases new batch of documents, images, videos from Epstein files
-
Four memorable showdowns between Alcaraz and Djokovic

-
 Russian figure skating prodigy Valieva set for comeback -- but not at Olympics
Russian figure skating prodigy Valieva set for comeback -- but not at Olympics
-
Barcelona midfielder Lopez agrees contract extension

-
 Djokovic says 'keep writing me off' after beating Sinner in late-nighter
Djokovic says 'keep writing me off' after beating Sinner in late-nighter
-
US Justice Dept releasing new batch of Epstein files

-
 South Africa and Israel expel envoys in deepening feud
South Africa and Israel expel envoys in deepening feud
-
French eyewear maker in spotlight after presidential showing

-
 Olympic dream 'not over', Vonn says after crash
Olympic dream 'not over', Vonn says after crash
-
Brazil's Lula discharged after cataract surgery

-
 US Senate races to limit shutdown fallout as Trump-backed deal stalls
US Senate races to limit shutdown fallout as Trump-backed deal stalls
-
'He probably would've survived': Iran targeting hospitals in crackdown

-
 Djokovic stuns Sinner to set up Australian Open final with Alcaraz
Djokovic stuns Sinner to set up Australian Open final with Alcaraz
-
Mateta omitted from Palace squad to face Forest

-
 Gold, silver prices tumble as investors soothed by Trump's Fed pick
Gold, silver prices tumble as investors soothed by Trump's Fed pick
-
Trump attorney general orders arrest of ex-CNN anchor covering protests

-
 Djokovic 'pushed to the limit' in stunning late-night Sinner upset
Djokovic 'pushed to the limit' in stunning late-night Sinner upset
-
Tunisia's famed blue-and-white village threatened after record rains

-
 Top EU official voices 'shock' at Minneapolis violence
Top EU official voices 'shock' at Minneapolis violence
-
Kremlin says agreed to halt strikes on Kyiv until Sunday

-
 Carrick calls for calm after flying start to Man Utd reign
Carrick calls for calm after flying start to Man Utd reign
-
Djokovic to meet Alcaraz in Melbourne final after five-set marathon

-
 Italian officials to testify in trial over deadly migrant shipwreck
Italian officials to testify in trial over deadly migrant shipwreck
-
Iran says defence capabilities 'never' up for negotiation

-
 UN appeals for more support for flood-hit Mozambicans
UN appeals for more support for flood-hit Mozambicans
-
Lijnders urges Man City to pile pressure on Arsenal in title race

-
 Fulham sign Man City winger Oscar Bobb
Fulham sign Man City winger Oscar Bobb
-
Strasbourg's Argentine striker Panichelli sets sights on PSG, World Cup


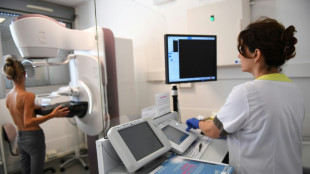
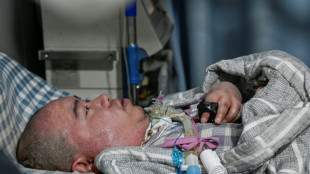
US to limit Covid boosters to over-65s or those at high risk
The United States will limit routine Covid-19 boosters to people over 65 or those at higher risk of serious illness, while requiring new placebo-controlled trials to justify vaccines for healthy individuals under that threshold, senior health officials said Tuesday.
In a letter to the prestigious New England Journal of Medicine, the Food and Drug Administration's Vinayak Prasad and Commissioner Martin Makary said the shift brought US policy more in line with European nations.
They described the initial rollout of Covid-19 vaccines as "a major scientific, medical, and regulatory accomplishment" -- but argued that the benefits of repeated boosters in low-risk individuals remained uncertain.
They contrasted the US approach with that of countries such as the United Kingdom, France and Germany, which limit booster recommendations to older adults and those with underlying conditions.
Going forward, the FDA believes it will continue to find the benefit-risk balance favorable for people over 65, and people over the age of six months with one or more underlying conditions.
However, "for all healthy persons -- those with no risk factors for severe Covid-19 -- between the ages of 6 months and 64 years, the FDA anticipates the need for randomized, controlled trial data," they said.
"The control group could receive a saline placebo."
They identified adults aged 50 to 64 as an ideal study population, and said trials should assess whether boosters reduce symptomatic illness, severe disease, hospitalization, and death.
Makary and Prasad also sought to reassure those worried about losing access to vaccines under the new framework.
The Centers for Disease Control and Prevention's definition of risk factors is "vast, including obesity and even mental health conditions such as depression," they wrote, adding that between 100 million and 200 million Americans would likely still qualify under this guidance.
Prasad, a hematologist-oncologist who now leads the FDA's Center for Biologics Evaluation and Research (CBER), rose to prominence during the pandemic for publicly questioning the widespread use of boosters.
M.O.Allen--AT