-
 Iran's Khamenei likens protests to 'coup', warns of regional war
Iran's Khamenei likens protests to 'coup', warns of regional war
-
New Epstein accuser claims sexual encounter with ex-prince Andrew: report

-
 Italy's extrovert Olympic icon Alberto Tomba insists he is 'shy guy'
Italy's extrovert Olympic icon Alberto Tomba insists he is 'shy guy'
-
Chloe Kim goes for unprecedented snowboard halfpipe Olympic treble

-
 Pakistan combing for perpetrators after deadly separatist attacks
Pakistan combing for perpetrators after deadly separatist attacks
-
Israel partially reopens Gaza's Rafah crossing

-
 Iran declares European armies 'terrorist groups' after IRGC designation
Iran declares European armies 'terrorist groups' after IRGC designation
-
Snowstorm disrupts travel in southern US as blast of icy weather widens

-
 Denmark's Andresen swoops to win Cadel Evans Road Race
Denmark's Andresen swoops to win Cadel Evans Road Race
-
Volkanovski beats Lopes in rematch to defend UFC featherweight title

-
 Sea of colour as Malaysia's Hindus mark Thaipusam with piercings and prayer
Sea of colour as Malaysia's Hindus mark Thaipusam with piercings and prayer
-
Exiled Tibetans choose leaders for lost homeland

-
 Afghan returnees in Bamiyan struggle despite new homes
Afghan returnees in Bamiyan struggle despite new homes
-
Mired in economic trouble, Bangladesh pins hopes on election boost

-
 Chinese cash in jewellery at automated gold recyclers as prices soar
Chinese cash in jewellery at automated gold recyclers as prices soar
-
Israel to partially reopen Gaza's Rafah crossing

-
 'Quiet assassin' Rybakina targets world number one after Melbourne win
'Quiet assassin' Rybakina targets world number one after Melbourne win
-
Deportation raids drive Minneapolis immigrant family into hiding

-
 Nvidia boss insists 'huge' investment in OpenAI on track
Nvidia boss insists 'huge' investment in OpenAI on track
-
'Immortal' Indian comics keep up with changing times

-
 With Trump mum, last US-Russia nuclear pact set to end
With Trump mum, last US-Russia nuclear pact set to end
-
In Sudan's old port of Suakin, dreams of a tourism revival

-
 Narco violence dominates as Costa Rica votes for president
Narco violence dominates as Costa Rica votes for president
-
Snowstorm barrels into southern US as blast of icy weather widens

-
 LA Olympic chief 'deeply regrets' flirty Maxwell emails in Epstein files
LA Olympic chief 'deeply regrets' flirty Maxwell emails in Epstein files
-
Rose powers to commanding six-shot lead at Torrey Pines

-
 Barca wasteful but beat Elche to extend Liga lead
Barca wasteful but beat Elche to extend Liga lead
-
Konate cut short compassionate leave to ease Liverpool injury crisis

-
 Separatist attacks in Pakistan kill 33, dozens of militants dead
Separatist attacks in Pakistan kill 33, dozens of militants dead
-
Dodgers manager Roberts says Ohtani won't pitch in Classic

-
 Arsenal stretch Premier League lead as Chelsea, Liverpool stage comebacks
Arsenal stretch Premier League lead as Chelsea, Liverpool stage comebacks
-
Korda defies cold and wind to lead LPGA opener

-
 New head of US mission in Venezuela arrives as ties warm
New head of US mission in Venezuela arrives as ties warm
-
Barca triumph at Elche to extend Liga lead

-
 Ekitike, Wirtz give Liverpool sight of bright future in Newcastle win
Ekitike, Wirtz give Liverpool sight of bright future in Newcastle win
-
West Indies 'tick boxes' in shortened T20 against South Africa

-
 Chelsea have something 'special' says Rosenior
Chelsea have something 'special' says Rosenior
-
De Zerbi 'ready to go to war' to solve Marseille troubles

-
 Hornets hold off Wemby's Spurs for sixth NBA win in a row
Hornets hold off Wemby's Spurs for sixth NBA win in a row
-
Moyes blasts killjoy booking after Everton's late leveller

-
 Ex-prince Andrew again caught up in Epstein scandal
Ex-prince Andrew again caught up in Epstein scandal
-
Bayern held at Hamburg to open door for Dortmund

-
 Atletico stumble to draw at Levante, Villarreal held
Atletico stumble to draw at Levante, Villarreal held
-
Chelsea stage impressive fightback to beat West Ham

-
 Arsenal stretch Premier League lead, Chelsea fightback breaks Hammers' hearts
Arsenal stretch Premier League lead, Chelsea fightback breaks Hammers' hearts
-
Napoli edge Fiorentina as injury crisis deepens

-
 How Lego got swept up in US-Mexico trade frictions
How Lego got swept up in US-Mexico trade frictions
-
UK rights campaigner Tatchell arrested at pro-Palestinian protest

-
 Iran says progress made towards US talks despite attack jitters
Iran says progress made towards US talks despite attack jitters
-
'Empowering': Ireland's first female sumo wrestler blazes a trail


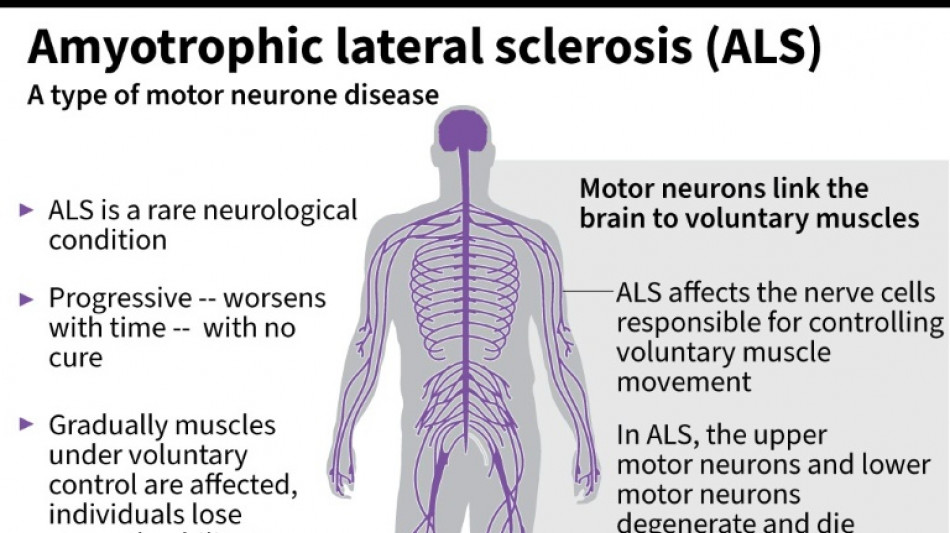
US company withdraws ALS drug after it fails in trial
Amylyx Pharmaceuticals announced Thursday it was withdrawing its approved treatment against the deadly neurodegenerative disease ALS after clinical data found no evidence the drug worked.
In a statement, the US company said it would discontinue its market authorizations for Relyvrio/Albrioza, using the brand names of the medicine in the US and Canadian markets.
"While this is a difficult moment for the ALS community, we reached this path forward in partnership with the stakeholders who will be impacted and in line with our steadfast commitment to people living with ALS and other neurodegenerative diseases," said the company's co-CEOs Joshua Cohen and Justin Klee in a statement.
The company also said it was reducing its workforce "by approximately 70 percent" as it focused on another experimental drug for use against ALS, and on repurposing Relyvrio for other conditions. It added it would continue to make Relyvrio available for patients who wish to keep using the treatment, through a "free drug program."
The news follows data from a clinical trial of 664 ALS patients announced in March, which found no significant differences in outcomes between those on the treatment group and those who received a placebo.
It was a big blow for patients with amyotrophic lateral sclerosis, sometimes called Lou Gehrig's disease after the famous baseball player, which devastates nerve cells in the brain and spinal cord.
ALS affects about two people per 100,000 every year, causing progressive loss of motor and cognitive function. Most patients die within five years of their diagnosis.
Relyvrio's approval by the US Food and Drug Administration in 2022 was controversial and based on the results of a single trial that involved just 137 participants.
The FDA itself noted there was "residual uncertainty about the evidence of effectiveness" -- but "given the serious and life-threatening nature of ALS and the substantial unmet need, this level of uncertainty is acceptable in this instance and consideration of these results in the context of regulatory flexibility is appropriate."
- Patient groups backed approval -
Advocacy groups also mounted a major campaign sending a petition to the FDA with tens of thousands of signatures urging approval. Once it became available, Amylyx reportedly announced an eye-watering list price of $158,000 per year in the US, drawing criticism.
Patient groups in Europe watched with desperation at the bureaucratic delays.
When the European Union drug watchdog later announced it was rejecting Relyvrio, the decision was slammed as "an affront" by angry French patients, who say they "don't have time to wait." France later relented, offering conditional approval in November.
"We commend Amylyx for pulling Relyvrio off the market, while still ensuring that people living with ALS can access the drug if they believe it is helping them," said the US-based ALS association, which had lobbied for the drug's approval and funded its research.
"Safe and potentially effective treatments can be made accessible rapidly until further research can confirm their efficacy," it added.
For now, there remain only a handful of treatments available.
Riluzole, FDA approved in 1995, prolongs life approximately three months. Edaravone, FDA approved in 2017, has been found to slow disease progression and improve survival.
And in 2023, the regulatory body approved tofersen, a gene therapy treatment that targets those ALS cases that are caused by mutations in the SOD1 gene.
Th.Gonzalez--AT