-
 Alcaraz sweeps past Djokovic to win 'dream' Australian Open
Alcaraz sweeps past Djokovic to win 'dream' Australian Open
-
Death toll from Swiss New Year bar fire rises to 41

-
 Alcaraz says Nadal inspired him to 'special' Australian Open title
Alcaraz says Nadal inspired him to 'special' Australian Open title
-
Pakistan seeks out perpetrators after deadly separatist attacks

-
 Ukraine war talks delayed to Wednesday, Zelensky says
Ukraine war talks delayed to Wednesday, Zelensky says
-
Djokovic says 'been a great ride' after Melbourne final loss

-
 Von Allmen storms to downhill win in final Olympic tune-up
Von Allmen storms to downhill win in final Olympic tune-up
-
Carlos Alcaraz: tennis history-maker with shades of Federer

-
 Alcaraz sweeps past Djokovic to win maiden Australian Open title
Alcaraz sweeps past Djokovic to win maiden Australian Open title
-
Israel says partially reopening Gaza's Rafah crossing

-
 French IT giant Capgemini to sell US subsidiary after row over ICE links
French IT giant Capgemini to sell US subsidiary after row over ICE links
-
Iran's Khamenei likens protests to 'coup', warns of regional war

-
 New Epstein accuser claims sexual encounter with ex-prince Andrew: report
New Epstein accuser claims sexual encounter with ex-prince Andrew: report
-
Italy's extrovert Olympic icon Alberto Tomba insists he is 'shy guy'

-
 Chloe Kim goes for unprecedented snowboard halfpipe Olympic treble
Chloe Kim goes for unprecedented snowboard halfpipe Olympic treble
-
Pakistan combing for perpetrators after deadly separatist attacks

-
 Israel partially reopens Gaza's Rafah crossing
Israel partially reopens Gaza's Rafah crossing
-
Iran declares European armies 'terrorist groups' after IRGC designation

-
 Snowstorm disrupts travel in southern US as blast of icy weather widens
Snowstorm disrupts travel in southern US as blast of icy weather widens
-
Denmark's Andresen swoops to win Cadel Evans Road Race

-
 Volkanovski beats Lopes in rematch to defend UFC featherweight title
Volkanovski beats Lopes in rematch to defend UFC featherweight title
-
Sea of colour as Malaysia's Hindus mark Thaipusam with piercings and prayer

-
 Exiled Tibetans choose leaders for lost homeland
Exiled Tibetans choose leaders for lost homeland
-
Afghan returnees in Bamiyan struggle despite new homes

-
 Mired in economic trouble, Bangladesh pins hopes on election boost
Mired in economic trouble, Bangladesh pins hopes on election boost
-
Chinese cash in jewellery at automated gold recyclers as prices soar

-
 Israel to partially reopen Gaza's Rafah crossing
Israel to partially reopen Gaza's Rafah crossing
-
'Quiet assassin' Rybakina targets world number one after Melbourne win

-
 Deportation raids drive Minneapolis immigrant family into hiding
Deportation raids drive Minneapolis immigrant family into hiding
-
Nvidia boss insists 'huge' investment in OpenAI on track

-
 'Immortal' Indian comics keep up with changing times
'Immortal' Indian comics keep up with changing times
-
With Trump mum, last US-Russia nuclear pact set to end

-
 In Sudan's old port of Suakin, dreams of a tourism revival
In Sudan's old port of Suakin, dreams of a tourism revival
-
Narco violence dominates as Costa Rica votes for president

-
 Snowstorm barrels into southern US as blast of icy weather widens
Snowstorm barrels into southern US as blast of icy weather widens
-
LA Olympic chief 'deeply regrets' flirty Maxwell emails in Epstein files

-
 Rose powers to commanding six-shot lead at Torrey Pines
Rose powers to commanding six-shot lead at Torrey Pines
-
BusinessHotels Launches AI Hotel Price Finder for Real-Time Rate Verification

-
 Sidekick Tools Announces Upcoming Depop OTL and WhatNot Follow Features Alongside AI Updates
Sidekick Tools Announces Upcoming Depop OTL and WhatNot Follow Features Alongside AI Updates
-
Remotify CEO Maria Sucgang Recognized as Tatler Gen.T Leader of Tomorrow

-
 The Blessing of Good Fortune Is Here: Own Equity in a Lithium Mining Company - Elektros Inc. - at a Bottom-Basement Discount, Right Here, Right Now
The Blessing of Good Fortune Is Here: Own Equity in a Lithium Mining Company - Elektros Inc. - at a Bottom-Basement Discount, Right Here, Right Now
-
Barca wasteful but beat Elche to extend Liga lead

-
 Konate cut short compassionate leave to ease Liverpool injury crisis
Konate cut short compassionate leave to ease Liverpool injury crisis
-
Separatist attacks in Pakistan kill 33, dozens of militants dead

-
 Dodgers manager Roberts says Ohtani won't pitch in Classic
Dodgers manager Roberts says Ohtani won't pitch in Classic
-
Arsenal stretch Premier League lead as Chelsea, Liverpool stage comebacks

-
 Korda defies cold and wind to lead LPGA opener
Korda defies cold and wind to lead LPGA opener
-
New head of US mission in Venezuela arrives as ties warm

-
 Barca triumph at Elche to extend Liga lead
Barca triumph at Elche to extend Liga lead
-
Ekitike, Wirtz give Liverpool sight of bright future in Newcastle win


Highly awaited Alzheimer's drug hit by delays
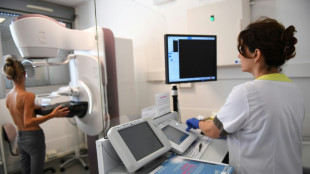
Eli Lilly's highly anticipated Alzheimer's drug has been held back for further review by regulators, the US pharmaceutical giant said Friday, in a blow for patients with the devastating brain disorder.
Donanemab has been found to slow cognitive decline in the early stages of the disease during a clinical trial -- but there was also a high rate of side effects, including deaths.
The Food and Drug Administration (FDA) "has informed Lilly it wants to further understand topics related to evaluating the safety and efficacy of donanemab," the company said in a statement Friday.
The regulator told the Indiana-based company it would convene a new meeting of experts, but hadn't provided a firm date. "As a result, the timing of expected FDA action on donanemab will be delayed beyond the first quarter of 2024."
"We are confident in donanemab's potential to offer very meaningful benefits to people with early symptomatic Alzheimer's disease," said Anne White, the company's executive vice president.
She added the FDA's decision to have a new meeting was "unexpected," but "We will work with the FDA and the stakeholders in the community to make that presentation and answer all questions."
Donanemab is an intravenously injected antibody that targets the build up beta-amyloid, a protein found in the brains of many patients with Alzheimer's.
Another anti-amyloid therapy called Leqembi, which was developed by Eisai of Japan and Biogen of Massachusetts, was granted full approval by the FDA last July and is now accessible through government-run health insurance for the elderly called Medicare.
- Slows decline, but risky -
In a paper published in the Journal of the American Medical Association last year, researchers found donanemab slowed cognitive and functional decline in patients who have early symptoms of the disease.
Forty-seven percent of those who received the drug showed no signs of cognitive decline after one year of treatment, compared to 29 percent who received a placebo.
Serious adverse events, including brain bleeds, occurred in 17.4 percent of those who received donanemab and 15.8 percent of those who received a placebo.
There were also four deaths: three in the donanemab group and one in the placebo group, but all the fatalities were considered a result of the treatment they received.
The trial recruited participants aged 60 to 85 with early symptomatic Alzheimer's, either mild cognitive impairment or Alzheimer's disease with mild dementia.
The news comes after the first Alzheimer's drug to be approved was pulled from the market in January.
The FDA awarded accelerated approval to Aduhelm in June 2021, a decision that was contentious at the time because the agency overruled its own independent advisors, who found there was insufficient evidence of benefit.
Biogen, which co-developed Aduhelm with Eisai, said it was discontinuing Aduhelm to focus its efforts of Leqembi.
Alzheimer's is the most common form of dementia. More than one in nine people over 65 develop the condition, which worsens over time, robbing them of their memories and independence, according to the US Alzheimer's Association.
R.Garcia--AT