
-
 US Justice Dept releasing new batch of Epstein files
US Justice Dept releasing new batch of Epstein files
-
South Africa and Israel expel envoys in deepening feud

-
 French eyewear maker in spotlight after presidential showing
French eyewear maker in spotlight after presidential showing
-
Olympic dream 'not over', Vonn says after crash

-
 Brazil's Lula discharged after cataract surgery
Brazil's Lula discharged after cataract surgery
-
US Senate races to limit shutdown fallout as Trump-backed deal stalls

-
 'He probably would've survived': Iran targeting hospitals in crackdown
'He probably would've survived': Iran targeting hospitals in crackdown
-
Djokovic stuns Sinner to set up Australian Open final with Alcaraz

-
 Mateta omitted from Palace squad to face Forest
Mateta omitted from Palace squad to face Forest
-
Gold, silver prices tumble as investors soothed by Trump's Fed pick

-
 Trump attorney general orders arrest of ex-CNN anchor covering protests
Trump attorney general orders arrest of ex-CNN anchor covering protests
-
Djokovic 'pushed to the limit' in stunning late-night Sinner upset

-
 Tunisia's famed blue-and-white village threatened after record rains
Tunisia's famed blue-and-white village threatened after record rains
-
Top EU official voices 'shock' at Minneapolis violence

-
 Kremlin says agreed to halt strikes on Kyiv until Sunday
Kremlin says agreed to halt strikes on Kyiv until Sunday
-
Carrick calls for calm after flying start to Man Utd reign

-
 Djokovic to meet Alcaraz in Melbourne final after five-set marathon
Djokovic to meet Alcaraz in Melbourne final after five-set marathon
-
Italian officials to testify in trial over deadly migrant shipwreck

-
 Iran says defence capabilities 'never' up for negotiation
Iran says defence capabilities 'never' up for negotiation
-
UN appeals for more support for flood-hit Mozambicans

-
 Lijnders urges Man City to pile pressure on Arsenal in title race
Lijnders urges Man City to pile pressure on Arsenal in title race
-
Fulham sign Man City winger Oscar Bobb

-
 Strasbourg's Argentine striker Panichelli sets sights on PSG, World Cup
Strasbourg's Argentine striker Panichelli sets sights on PSG, World Cup
-
Jesus 'made love': Colombian president irks Christians with steamy claim

-
 IAEA board meets over Ukraine nuclear safety concerns
IAEA board meets over Ukraine nuclear safety concerns
-
Eurozone growth beats 2025 forecasts despite Trump woes

-
 Israel to partially reopen Gaza's Rafah crossing on Sunday
Israel to partially reopen Gaza's Rafah crossing on Sunday
-
Dutch PM-elect Jetten says not yet time to talk to Putin

-
 Social media fuels surge in UK men seeking testosterone jabs
Social media fuels surge in UK men seeking testosterone jabs
-
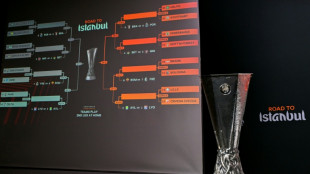
Forest face Fenerbahce, Celtic draw Stuttgart in Europa League play-offs
-
 US speed queen Vonn crashes at Crans-Montana, one week before Olympics
US speed queen Vonn crashes at Crans-Montana, one week before Olympics
-
Trump nominates former US Fed official as next central bank chief

-
 Alcaraz defends controversial timeout after beaten Zverev fumes
Alcaraz defends controversial timeout after beaten Zverev fumes
-
New Dutch government pledges ongoing Ukraine support

-
 Newcastle still coping with fallout from Isak exit, says Howe
Newcastle still coping with fallout from Isak exit, says Howe
-
Chad, France eye economic cooperation as they reset strained ties

-
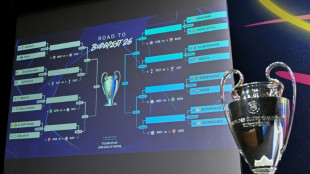 Real Madrid to play Benfica, PSG face Monaco in Champions League play-offs
Real Madrid to play Benfica, PSG face Monaco in Champions League play-offs
-
Everton winger Grealish set to miss rest of season in World Cup blow

-
 Trump brands Minneapolis nurse killed by federal agents an 'agitator'
Trump brands Minneapolis nurse killed by federal agents an 'agitator'
-
Arteta focuses on the positives despite Arsenal stumble

-
 Fijian Drua sign France international back Vakatawa
Fijian Drua sign France international back Vakatawa
-
Kevin Warsh, a former Fed 'hawk' now in tune with Trump

-
 Zverev rails at Alcaraz timeout in 'one of the best battles ever'
Zverev rails at Alcaraz timeout in 'one of the best battles ever'
-
Turkey leads Iran diplomatic push as Trump softens strike threat

-
 Zelensky backs energy ceasefire, Russia bombs Ukraine despite Trump intervention
Zelensky backs energy ceasefire, Russia bombs Ukraine despite Trump intervention
-
'Superman' Li Ka-shing, Hong Kong billionaire behind Panama ports deal

-
 Skiing great Lindsey Vonn crashes at Crans-Montana, one week before Olympics
Skiing great Lindsey Vonn crashes at Crans-Montana, one week before Olympics
-
Slot warns Liverpool 'can't afford mistakes' in top-four scrap

-
 Paris show by late Martin Parr views his photos through political lens
Paris show by late Martin Parr views his photos through political lens
-
'Believing' Alcaraz outlasts Zverev in epic to reach maiden Melbourne final

| RBGPF | 1.65% | 83.78 | $ | |
| SCS | 0.12% | 16.14 | $ | |
| RYCEF | -2.69% | 16 | $ | |
| NGG | -0.33% | 84.77 | $ | |
| CMSC | -0.02% | 23.69 | $ | |
| AZN | 0.38% | 92.94 | $ | |
| GSK | 1.03% | 51.18 | $ | |
| BP | 0.09% | 38.075 | $ | |
| BTI | -0.47% | 59.93 | $ | |
| RIO | -3.08% | 92.29 | $ | |
| VOD | -0.55% | 14.63 | $ | |
| BCE | 0.1% | 25.51 | $ | |
| JRI | 0.23% | 12.985 | $ | |
| BCC | -1.31% | 79.132 | $ | |
| RELX | -1.25% | 35.72 | $ | |
| CMSD | 0.05% | 24.073 | $ |

Unlearn Introduces TrialPioneer, an AI-powered Workspace to Strengthen Upstream Trial Planning
The workspace was designed to accelerate and strengthen decision-making in upstream trial planning
SAN FRANCISCO, CA / ACCESS Newswire / January 28, 2026 / Unlearn, a leader in AI solutions for clinical development, today announced the launch of TrialPioneer, an AI-powered workspace that helps clinical development teams accelerate and strengthen decision-making in upstream trial planning-optimizing study designs to maximize the probability of success with speed and scientific rigor.
Upstream trial planning is where teams make and refine the design choices that shape a study's feasibility and likelihood of success. These choices include specifying endpoints, eligibility criteria, target populations, and key assumptions about outcomes, often under significant uncertainty. As evidence evolves, internal strategy shifts, and cross-functional review cycles progress, planning workflows can become fragmented, with scattered literature searches, disconnected analyses, and one-off simulations that are hard to reproduce or revisit as the design changes.
TrialPioneer addresses this gap with a purpose-built workspace that brings together evidence, assumptions, historical benchmarks, and scenario evaluations in one workflow to optimize study designs. By making assumptions explicit and traceable, and enabling comparisons across design scenarios, TrialPioneer helps study teams evaluate trade-offs earlier and align on the strongest path forward. Unlike workflows where evidence review, analyses, and simulations live in separate systems, TrialPioneer keeps the decision context connected as trial designs evolve.
By grounding assumptions in historical evidence and disease-relevant context, and streamlining evidence review and scenario setup, TrialPioneer helps clinical development teams align earlier and faster on key trade-offs, build confidence in design choices ahead of governance reviews, and reduce late-stage rework when planning decisions are revisited.
How TrialPioneer Works
TrialPioneer integrates three core capabilities into a single workflow:
Scout - AI-powered precedent review:
Continuously structures and summarizes scientific and regulatory precedent from sources such as PubMed, ClinicalTrials.gov, and FDA databases-so teams can easily reference what's been done in all publicly available trials.
Hindsight - Historical benchmark exploration:
Enables teams to compare clinical and statistical assumptions against what's been observed in harmonized patient-level clinical trial and real-world datasets-supporting benchmark-driven planning and earlier.
SimLab - On demand trial simulations:
Allows teams to model and compare trial design scenarios to explore what could happen across endpoints, eligibility criteria, and sample size-producing explainable outputs tied to underlying assumptions and evidence.
"Clinical development leaders are under pressure to move faster while maintaining scientific rigor," said Steve Herne, CEO of Unlearn. "TrialPioneer was designed to help teams pressure test trial design decisions earlier-linking evidence, assumptions, benchmarks, and scenario outputs in one workflow as designs evolve."
"The ability to summarize key features of past trials, construct disease-specific cohorts, and run clinical trial scenarios on demand changes how teams make study design and protocol decisions," said Dr. Robert Lenz, strategic advisor to Unlearn. "Most teams spend weeks going back and forth on questions that TrialPioneer can help answer in real time. Because the work is saved and traceable, you're building institutional knowledge instead of starting from scratch every time."
About Unlearn
Unlearn exists to transform clinical development by making every trial smarter. Partnering with pharmaceutical and biotechnology companies, Unlearn harnesses data, AI, and digital twins to enable faster, more robust studies and clearer decision-making across clinical development. With a science-first approach and deep regulatory engagement-including EMA qualification and FDA support-Unlearn brings unmatched scientific credibility to applying AI in clinical trials.
Industry analysts, pharmaceutical, and biotechnology teams interested in learning more about TrialPioneer can visit URL to request additional information.
Media Contact:
Heather D'Angelo
[email protected]
SOURCE: Unlearn AI
View the original press release on ACCESS Newswire
S.Jackson--AT
