
-
 Iran says defence capabilities 'never' up for negotiation
Iran says defence capabilities 'never' up for negotiation
-
UN appeals for more support for flood-hit Mozambicans

-
 Lijnders urges Man City to pile pressure on Arsenal in title race
Lijnders urges Man City to pile pressure on Arsenal in title race
-
Fulham sign Man City winger Oscar Bobb

-
 Strasbourg's Argentine striker Panichelli sets sights on PSG, World Cup
Strasbourg's Argentine striker Panichelli sets sights on PSG, World Cup
-
Jesus 'made love': Colombian president irks Christians with steamy claim

-
 IAEA board meets over Ukraine nuclear safety concerns
IAEA board meets over Ukraine nuclear safety concerns
-
Eurozone growth beats 2025 forecasts despite Trump woes

-
 Israel to partially reopen Gaza's Rafah crossing on Sunday
Israel to partially reopen Gaza's Rafah crossing on Sunday
-
Dutch PM-elect Jetten says not yet time to talk to Putin

-
 Social media fuels surge in UK men seeking testosterone jabs
Social media fuels surge in UK men seeking testosterone jabs
-
Forest face Fenerbahce, Celtic draw Stuttgart in Europa League play-offs
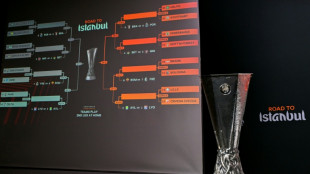
-
 US speed queen Vonn crashes at Crans-Montana, one week before Olympics
US speed queen Vonn crashes at Crans-Montana, one week before Olympics
-
Trump nominates former US Fed official as next central bank chief

-
 Alcaraz defends controversial timeout after beaten Zverev fumes
Alcaraz defends controversial timeout after beaten Zverev fumes
-
New Dutch government pledges ongoing Ukraine support

-
 Newcastle still coping with fallout from Isak exit, says Howe
Newcastle still coping with fallout from Isak exit, says Howe
-
Chad, France eye economic cooperation as they reset strained ties

-
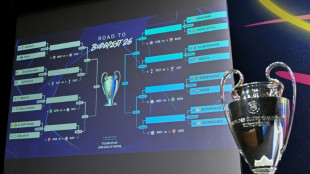 Real Madrid to play Benfica, PSG face Monaco in Champions League play-offs
Real Madrid to play Benfica, PSG face Monaco in Champions League play-offs
-
Everton winger Grealish set to miss rest of season in World Cup blow

-
 Trump brands Minneapolis nurse killed by federal agents an 'agitator'
Trump brands Minneapolis nurse killed by federal agents an 'agitator'
-
Arteta focuses on the positives despite Arsenal stumble

-
 Fijian Drua sign France international back Vakatawa
Fijian Drua sign France international back Vakatawa
-
Kevin Warsh, a former Fed 'hawk' now in tune with Trump

-
 Zverev rails at Alcaraz timeout in 'one of the best battles ever'
Zverev rails at Alcaraz timeout in 'one of the best battles ever'
-
Turkey leads Iran diplomatic push as Trump softens strike threat

-
 Zelensky backs energy ceasefire, Russia bombs Ukraine despite Trump intervention
Zelensky backs energy ceasefire, Russia bombs Ukraine despite Trump intervention
-
'Superman' Li Ka-shing, Hong Kong billionaire behind Panama ports deal

-
 Skiing great Lindsey Vonn crashes at Crans-Montana, one week before Olympics
Skiing great Lindsey Vonn crashes at Crans-Montana, one week before Olympics
-
Slot warns Liverpool 'can't afford mistakes' in top-four scrap

-
 Paris show by late Martin Parr views his photos through political lens
Paris show by late Martin Parr views his photos through political lens
-
'Believing' Alcaraz outlasts Zverev in epic to reach maiden Melbourne final

-
 Artist chains up thrashing robot dog to expose AI fears
Artist chains up thrashing robot dog to expose AI fears
-
Alcaraz outlasts Zverev in epic to reach maiden Australian Open final

-
 French PM forces final budget through parliament
French PM forces final budget through parliament
-
French-Nigerian artists team up to craft future hits

-
 Dutch watchdog launches Roblox probe over 'risks to children'
Dutch watchdog launches Roblox probe over 'risks to children'
-
Trump brands Minneapolis nurse shot dead by federal agents an 'agitator'

-
 Israel says killed 'three terrorists' in Gaza
Israel says killed 'three terrorists' in Gaza
-
After Trump-fueled brawls, Canada-US renew Olympic hockey rivalry

-
 Eileen Gu - Olympic champion who bestrides rivals US, China
Eileen Gu - Olympic champion who bestrides rivals US, China
-
Trump, first lady attend premier of multimillion-dollar 'Melania' documentary

-
 US Senate eyes funding deal vote as government shutdown looms
US Senate eyes funding deal vote as government shutdown looms
-
Cuddly Olympics mascot facing life or death struggle in the wild

-
 UK schoolgirl game character Amelia co-opted by far-right
UK schoolgirl game character Amelia co-opted by far-right
-
Anger as bid to ramp up Malaysia's football fortunes backfires

-
 Panama court annuls Hong Kong firm's canal port concession
Panama court annuls Hong Kong firm's canal port concession
-
Pioneer African Olympic skier returns to Sarajevo slopes for documentary

-
 Trump threatens tariffs on nations selling oil to Cuba
Trump threatens tariffs on nations selling oil to Cuba
-
From fragile youngster to dominant star, Sabalenka chases more glory


Moderna Announces Strategic Collaboration with Recordati to Globally Commercialize Investigational Propionic Acidemia Therapeutic (mRNA-3927)
Moderna will continue to lead clinical development and manufacturing for mRNA-3927
Moderna to receive up to $160 million in upfront and near-term development and regulatory milestones, in addition to commercial and sales milestones and tiered royalties on net sales
CAMBRIDGE, MA / ACCESS Newswire / January 29, 2026 / Moderna, Inc. (NASDAQ:MRNA) today announced a strategic collaboration with Recordati to advance Moderna's investigational propionic acidemia (PA) therapeutic, mRNA-3927, through the final stages of clinical development and, upon approval, global commercialization. Recordati, based in Milan, Italy, is an international pharmaceutical group that provides treatments across specialty and primary care, and rare diseases, including PA. Through this agreement, Moderna will continue to lead the clinical development of mRNA-3927 through approval and Recordati will lead commercialization.
"We are proud to partner with Recordati in a joint mission to improve the lives of people living with propionic acidemia," said Stéphane Bancel, Chief Executive Officer of Moderna. "Recordati brings deep rare disease commercial expertise and an established global commercial infrastructure in propionic acidemia that will help us accelerate the benefit of mRNA-3927 upon approval."
"Propionic acidemia is a serious rare disease with a significant unmet medical need due to the lack of disease modifying treatment options to date. We look forward to partnering with Moderna," said Rob Koremans, Chief Executive Officer of Recordati. "Their experience in applying innovative mRNA technology, combined with our experience in rare metabolic disorders and strong established commercial infrastructure, positions us well to advance this potential therapy together to serve patients. We are encouraged by the clinical data and look forward to the pivotal readout expected in 2026. This deal strengthens our development portfolio and builds on our heritage in the metabolic field."
Under the terms of the agreement, Moderna will receive an upfront payment of $50 million and up to an additional $110 million in near-term development and regulatory milestones, in addition to commercial and sales milestones and tiered royalties on net sales. The transaction is subject to customary closing conditions, including U.S. antitrust clearance which is expected within 30 days from the relevant filing.
mRNA-3927 is currently being evaluated in a registrational study that has reached target enrollment. The Company expects a potential data readout in 2026.
About propionic acidemia (PA)
Propionic acidemia is a rare, serious, inherited metabolic disorder with significant morbidity and mortality, affecting 1 in 100,000-150,000 individuals worldwide. PA is caused by pathogenic variants in the propionyl-coenzyme A carboxylase (PCC) α or β subunits (PCCA and PCCB genes, respectively), leading to PCC deficiency and subsequent accumulation of toxic metabolites. PA is characterized by recurrent life-threatening metabolic decompensation events (MDEs) and multisystemic complications. Currently, there are no effective therapies for PA that target the underlying root cause of the disease.
About mRNA-3927
mRNA-3927 is an investigational novel mRNA-based therapeutic agent that is composed of two mRNAs encoding for normal human PCCA and PCCB subunits. Intravenous (IV) administration of mRNA-3927 is intended to restore functional PCC enzymes in patients with PA.
Interim data from a first-in-human, phase 1/2, open-label, dose optimization study and extension study evaluating the safety and efficacy of mRNA-3927 indicate early signs of potential clinical benefit and demonstrate that mRNA-3927 has infrequent treatment-limiting side effects.
About Moderna
Moderna is a pioneer and leader in the field of mRNA medicine. Through the advancement of its technology platform, Moderna is reimagining how medicines are made to transform how we treat and prevent diseases. Since its founding, Moderna's mRNA platform has enabled the development of vaccines and therapeutics across infectious diseases, cancer, rare diseases and more.
With a global team and a unique culture, driven by the company's values and mindsets, Moderna's mission is to deliver the greatest possible impact to people through mRNA medicines. For more information about Moderna, please visit modernatx.com and connect with us on X, Facebook, Instagram, YouTube and LinkedIn.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including statements regarding: Moderna's collaboration with Recordati to commercialize its PA therapy; Moderna's clinical development of mRNA-3927; timing of an expected pivotal data readout in 2026; the potential for regulatory approval and commercialization of mRNA-3927; potential payments, milestones and royalties under the collaboration agreement; and expected closing of the transaction and customary closing conditions, including U.S. antitrust clearance. In some cases, forward-looking statements can be identified by terminology such as "will," "may," "should," "could," "expects," "intends," "plans," "aims," "anticipates," "believes," "estimates," "predicts," "potential," "continue," or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. The forward-looking statements in this press release are neither promises nor guarantees, and you should not place undue reliance on these forward-looking statements because they involve known and unknown risks, uncertainties, and other factors, many of which are beyond Moderna's control and which could cause actual results to differ materially from those expressed or implied by these forward-looking statements. These risks, uncertainties, and other factors include, among others, those risks and uncertainties described under the heading "Risk Factors" in Moderna's Annual Report on Form 10-K for the fiscal year ended December 31, 2024, filed with the U.S. Securities and Exchange Commission (SEC), and in subsequent filings made by Moderna with the SEC, which are available on the SEC's website at www.sec.gov. Except as required by law, Moderna disclaims any intention or responsibility for updating or revising any forward-looking statements contained in this press release in the event of new information, future developments or otherwise. These forward-looking statements are based on Moderna's current expectations and speak only as of the date of this press release.
Moderna Contacts
Media:
Chris Ridley
Head of Global Media Relations
+1 617-800-3651
[email protected]
Investors:
Lavina Talukdar
Senior Vice President & Head of Investor Relations
+1 617-209-5834
[email protected]
SOURCE: Moderna, Inc.
View the original press release on ACCESS Newswire
H.Romero--AT

