
-
 Native Americans on high alert over Minneapolis crackdown
Native Americans on high alert over Minneapolis crackdown
-
Dallas deals Davis to Wizards in blockbuster NBA deal: report

-
 Russia 'no longer bound' by nuclear arms limits as treaty with US ends
Russia 'no longer bound' by nuclear arms limits as treaty with US ends
-
Panama hits back after China warns of 'heavy price' in ports row

-
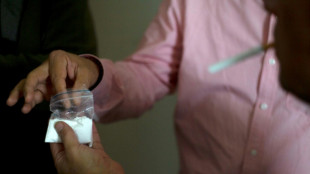 Strike kills guerrillas as US, Colombia agree to target narco bosses
Strike kills guerrillas as US, Colombia agree to target narco bosses
-
Wildfire smoke kills more than 24,000 Americans a year: study

-
 Telegram founder slams Spain PM over under-16s social media ban
Telegram founder slams Spain PM over under-16s social media ban
-
Curling kicks off sports programme at 2026 Winter Olympics

-
 Preventative cholera vaccination resumes as global supply swells: WHO
Preventative cholera vaccination resumes as global supply swells: WHO
-
Wales' Macleod ready for 'physical battle' against England in Six Nations

-
 Xi calls for 'mutual respect' with Trump, hails ties with Putin
Xi calls for 'mutual respect' with Trump, hails ties with Putin
-
'All-time great': Maye's ambitions go beyond record Super Bowl bid

-
 Shadow over Vonn as Shiffrin, Odermatt headline Olympic skiing
Shadow over Vonn as Shiffrin, Odermatt headline Olympic skiing
-
US seeks minerals trade zone in rare Trump move with allies

-
 Ukraine says Abu Dhabi talks with Russia 'substantive and productive'
Ukraine says Abu Dhabi talks with Russia 'substantive and productive'
-
Brazil mine disaster victims in London to 'demand what is owed'

-
 AI-fuelled tech stock selloff rolls on
AI-fuelled tech stock selloff rolls on
-
Russia vows to act 'responsibly' as nuclear pact ends with US

-
 White says time at Toulon has made him a better Scotland player
White says time at Toulon has made him a better Scotland player
-
Washington Post announces 'painful' job cuts

-
 All lights are go for Jalibert, says France's Dupont
All lights are go for Jalibert, says France's Dupont
-
Artist rubs out Meloni church fresco after controversy

-
 Palestinians in Egypt torn on return to a Gaza with 'no future'
Palestinians in Egypt torn on return to a Gaza with 'no future'
-
US removing 700 immigration officers from Minnesota

-
 Who is behind the killing of late ruler Gaddafi's son, and why now?
Who is behind the killing of late ruler Gaddafi's son, and why now?
-
Coach Thioune tasked with saving battling Bremen

-
 Russia vows to act 'responsibly' once nuclear pact with US ends
Russia vows to act 'responsibly' once nuclear pact with US ends
-
Son of Norway's crown princess admits excesses but denies rape

-
 US calls for minerals trade zone in rare move with allies
US calls for minerals trade zone in rare move with allies
-
Vowles dismisses Williams 2026 title hopes as 'not realistic'

-
 'Dinosaur' Glenn chasing skating gold in first Olympics
'Dinosaur' Glenn chasing skating gold in first Olympics
-
Gaza health officials say strikes kill 23 after Israel says shots wounded officer

-
 Italy foils Russian cyberattacks targeting Olympics
Italy foils Russian cyberattacks targeting Olympics
-
Stocks stabilise after Wall St AI-fuelled sell-off

-
 Figure skating favourite Malinin feeling 'the pressure' in Milan
Figure skating favourite Malinin feeling 'the pressure' in Milan
-
Netflix film probes conviction of UK baby killer nurse

-
 Timber hopes League Cup can be catalyst for Arsenal success
Timber hopes League Cup can be catalyst for Arsenal success
-
China calls EU 'discriminatory' over probe into energy giant Goldwind

-
 Sales warning slams Ozempic maker Novo Nordisk's stock
Sales warning slams Ozempic maker Novo Nordisk's stock
-
Can Vonn defy ACL rupture to win Olympic medal?

-
 Breakthrough or prelude to attack? What we know about Iran-US talks
Breakthrough or prelude to attack? What we know about Iran-US talks
-
German far-right MP detained over alleged Belarus sanctions breach

-
 MSF says its hospital in South Sudan hit by government air strike
MSF says its hospital in South Sudan hit by government air strike
-
Merz heads to Gulf as Germany looks to diversify trade ties

-
 Selection process for future Olympic hosts set for reform
Selection process for future Olympic hosts set for reform
-
Serbian minister on trial over Trump-linked hotel plan

-
 UK PM says Mandelson 'lied', regrets appointing him US envoy
UK PM says Mandelson 'lied', regrets appointing him US envoy
-
Cochran-Siegle tops first Olympic downhill training

-
 Gaza health officials say strikes kill 21 after Israel says shots wounded officer
Gaza health officials say strikes kill 21 after Israel says shots wounded officer
-
Injured Vonn's Olympic bid is 'inspirational', ski stars say

| SCS | 0.12% | 16.14 | $ | |
| RYCEF | -2.1% | 16.65 | $ | |
| CMSC | -0.51% | 23.54 | $ | |
| RBGPF | 0.12% | 82.5 | $ | |
| RELX | -1.65% | 30.015 | $ | |
| CMSD | -0.5% | 23.82 | $ | |
| NGG | 2.37% | 88.32 | $ | |
| RIO | -0.47% | 95.92 | $ | |
| GSK | 7.05% | 57.383 | $ | |
| BCE | 1.12% | 26.395 | $ | |
| VOD | 2.97% | 15.717 | $ | |
| BCC | 5.5% | 89.87 | $ | |
| AZN | 1.98% | 188.04 | $ | |
| BTI | -0.21% | 61.74 | $ | |
| JRI | 0.04% | 13.125 | $ | |
| BP | 1.51% | 39.415 | $ |

Medicus Pharma Ltd. Receives Positive Feedback From the Food and Drug Adminstration (FDA) Type C Meeting Supporting the Development of Skinject
The FDA agrees that the Company may follow 505(b)(2) regulatory pathway to non-invasively treat basal cell carcinoma (BCC) of the skin using dissolvable Doxorubicin-containing Microneedle arrays (D-MNA) representing ~$2 billion in potential market opportunity
The Company plans to complete patient recruitment for SKNJCT-003 before the end of Q4 2025 and to request End-of-Phase 2 (EOP2) with the FDA in Q1 2026
The FDA agrees that the Company may follow 505(b)(2) regulatory pathway to non-invasively treat basal cell carcinoma (BCC) of the skin using dissolvable Doxorubicin-containing Microneedle arrays (D-MNA) representing ~$2 billion in potential market opportunity
The Company plans to complete patient recruitment for SKNJCT-003 before the end of Q4 2025 and to request End-of-Phase 2 (EOP2) with the FDA in Q1 2026
PHILADELPHIA, PENNSYLVANIA / ACCESS Newswire / September 29, 2025 / Medicus Pharma Ltd. (NASDAQ:MDCX) ("Medicus" or the "Company"), a biotech/life sciences company focused on advancing the clinical development programs of novel and potentially disruptive therapeutics assets, is pleased to announce positive feedback from Type C meeting with United States Food and Drug Administration (FDA).
The purpose of the Type C meeting was to discuss the design, endpoints, and other key protocol elements for the clinical development of doxorubicin containing microneedle array (D-MNA) to non-invasively treat basal cell carcinoma (BCC) of the skin.
The FDA provided clarity and further alignment that a relative bioavailability study with the Company's D-MNA product to treat BCC administered under certain conditions could provide support towards establishing a clinical bridge for the 505(b)(2) regulatory pathway as well as help satisfy the bioavailability requirement of the Company's D-MNA product as stated in 21CFR320.21. More specifically, the FDA provided positive and constructive feedback on a number of topics for the continued development of Skinject including, but not limited to: the appropriate primary endpoint for the next study; proposed patient population definition, including tumor size limits, location restrictions, and histological confirmation requirements; proposed randomized, double-blind, placebo-controlled, study design for future studies that are intended to demonstrate the effectiveness of D-MNA in treating BCC; and current safety assessments for the proposed study.
In anticipation of future pivotal studies, the FDA also provided recommendations to continue to optimize formulation and microneedle design, integrate an adhesive layer to affix the microneedle system to the body, and incorporate the use of an applicator to consistently apply the product to the application site.
"Establishing 505(b)(2) as a regulatory pathway to bring to market our novel, non-invasive Skinject D-MNA treatment to cure BCC of the skin, is a game changer" stated Dr. Raza Bokhari, Medicus's Executive Chairman & CEO, "by leveraging existing doxorubicin safety data, we will not only realize substantial drug development cost savings but also time savings compared to a traditional full development program. As we plan to complete patient recruitment for SKNJCT-003 before the end this year and request an EOP2 meeting with the FDA in Q1 2026, our confidence is increasing that Skinject may become commercially viable sometime in 2027".
The Company is currently conducting a Phase 2 clinical study for SKNJCT-003 in nine (9) clinical sites across the United States which commenced randomizing patients in August 2024. In March 2025, the Company also announced a positively trending interim analysis for SKNJCT-003 demonstrating more than 60% clinical clearance. The interim analysis was conducted after more than 50% of the then-targeted 60 patients in the study were randomized. The findings of the interim analysis are preliminary and may or may not correlate with the findings of the study once completed. In April 2025, the investigational review board approved to increase the number of participants in SKNJCT-003 to ninety (90) subjects. The Company is expanding its trial sites in Europe and has randomized more than 75% of the ninety (90) participants expected to be randomized in the study.
The Company also has a clinical study (SKNJCT-004) currently underway in the United Arab Emirates (UAE). The study is expected to randomize thirty-six (36) patients in six (6) sites in the UAE. Cleveland Clinic Abu Dhabi (CCAD) is the principal investigator, along with Sheikh Shakbout Medical City (SSMC), Burjeel Medical City (BMC), Rashid Hospital (RH), Clemenceau Medical Center (CMC) and American Hospital of Dubai (AHD). Insights Research Organization and Solutions (IROS), a UAE-based contract research organization, is coordinating the clinical study for the Company. IROS is a M42 portfolio company.
In August 2025, the Company completed the acquisition of Antev, a UK-based late clinical stage biotech company, developing Teverelix, a next generation GnRH antagonist, as a first in market product for cardiovascular high-risk advanced prostate cancer patients and patients with first acute urinary retention relapse (AURr) episodes due to enlarged prostate.
Antev's flagship drug candidate is Teverelix trifluoroacetate (Teverelix TFA), a long-acting gonadotrophin-releasing hormone (GnRH) antagonist. Unlike GnRH agonists, which can cause an initial surge in testosterone levels, Teverelix directly suppresses sex hormone production without this surge, potentially reducing cardiovascular risks. This mechanism is particularly beneficial for patients with existing cardiovascular conditions. Teverelix is formulated as a microcrystalline suspension, allowing for sustained release and a six-week dosing interval, which may improve patient compliance and outcomes
For further information contact:
Carolyn Bonner, President and Acting Chief Financial Officer
(610) 636-0184
[email protected]
Anna Baran-Djokovic, SVP Investor Relations
(305) 615-9162
[email protected]
About Medicus Pharma Ltd.
Medicus Pharma Ltd. (Nasdaq:MDCX) is a biotech/life sciences company focused on accelerating the clinical development programs of novel and potentially disruptive therapeutics assets. The Company is actively engaged in multiple countries, spread over three continents.
SkinJect Inc. a wholly owned subsidiary of Medicus Pharma Ltd., is a development stage, life sciences company focused on commercializing novel, non-invasive treatment for basal cell skin cancer using a patented dissolvable microneedle patch to deliver a chemotherapeutic agent to eradicate tumors cells. The Company completed a phase 1 safety & tolerability study (SKNJCT-001) in March of 2021, which met its primary objective of safety and tolerability; the study also describes the efficacy of the investigational product D-MNA, with six (6) participants experiencing complete response on histological examination of the resected lesion. The Company is currently conducting a randomized, controlled, double-blind, multicenter clinical study (SKNJCT-003) in the United States and Europe. The Company has also commenced a randomized, controlled, double-blind, multicenter clinical study (SKNJCT-004) in the United Arab Emirates.
In August 2025, the Company announced its entry into a non-binding memorandum of understanding (the "MoU") with Helix Nanotechnologies, Inc. ("HelixNano"), a Boston Based biotech company focused on developing a proprietary advanced mRNA platform, in respect of their shared mutual interest in the development or commercial arrangement contemplated by the MoU. The MoU is non-binding and shall not be construed to obligate either party to proceed with a joint venture or any further development or commercial arrangement, unless and until definitive agreements are executed.
In August 2025, the Company completed the acquisition of Antev, a UK-based late clinical stage biotech company, developing Teverelix, a next generation GnRH antagonist, as a first in market product for cardiovascular high-risk advanced prostate cancer patients and patients with first acute urinary retention relapse (AURr) episodes due to enlarged prostate.
Antev's flagship drug candidate is Teverelix trifluoroacetate (Teverelix TFA), a long-acting gonadotrophin-releasing hormone (GnRH) antagonist. Unlike GnRH agonists, which can cause an initial surge in testosterone levels, Teverelix directly suppresses sex hormone production without this surge, potentially reducing cardiovascular risks. This mechanism is particularly beneficial for patients with existing cardiovascular conditions. Teverelix is formulated as a microcrystalline suspension, allowing for sustained release and a six-week dosing interval, which may improve patient compliance and outcomes
In September 2020, Antev completed a Phase 1 clinical trial in which Teverelix was shown to be well tolerated with no dose-limiting toxicities and demonstrated rapid testosterone suppression. The study included 48 healthy male volunteers. In February 2023, Antev also completed a Phase 2a study in fifty (50) patients with advanced prostate cancer (APC), where Teverelix achieved the primary endpoint of greater than 90% probability of castration levels of testosterone suppression (97.5%) but the secondary endpoint of maintaining this rate above 90% was not met with the probability dropping to 82.5% by Day 42.
In January 2023, the FDA, reviewed the Phase 1 and Phase 2a data and provided written guidance on Antev's proposed Phase 3 trial design for Teverelix. This milestone supports the Company's clinical plans to develop Teverelix as a treatment for advanced prostate cancer patients with increased cardiovascular risk.
In December 2023, FDA approved the Phase 2b study design in advanced prostate cancer covering 40 patients.
In November 2024, FDA approved the Phase 2b study design in acute urinary retention covering 390 patients.
Cautionary Notice on Forward-Looking Statements
Certain information in this news release constitutes "forward-looking information" under applicable securities laws. "Forward-looking information" is defined as disclosure regarding possible events, conditions or financial performance that is based on assumptions about future economic conditions and courses of action and includes, without limitation, the development of Teverelix and expectations concerning, and future outcomes relating to, the development, advancement and commercialization of Teverelix for AURr and high CV risk prostate cancer, and the potential market opportunities related thereto, the MOU, including the potential signing of definitive agreements between Medicus and HelixNano and the development of thermostable infectious diseases vaccines by combining HelixNano's proprietary mRNA vaccine platform with Medicus's proprietary microneedle array (MNA) delivery platform, the Company's aim to fast-track the clinical development program and convert the SKNJCT-003 exploratory clinical trial into a pivotal clinical trial, and approval from the FDA and the timing thereof, plans and expectations concerning, and future outcomes relating to, the development, advancement and commercialization of SkinJect through SKNJCT-003 and SKNJCT-004, and the potential market opportunities related thereto, the commencement of the SKNJCT-004 study and the potential results of and benefits of such study. Forward-looking statements are often but not always, identified by the use of such terms as "may", "on track", "aim", "might", "will", "will likely result", "could," "designed," "would", "should", "estimate", "plan", "project", "forecast", "intend", "expect", "anticipate", "believe", "seek", "continue", "target", "potential" or the negative and/or inverse of such terms or other similar expressions. These statements involve known and unknown risks, uncertainties and other factors, which may cause actual results, performance or achievements to differ materially from those expressed or implied by such statements, including those risk factors described in the Company's annual report on form 10-K for the year ended December 31, 2024 (the "Annual Report"), and in the Company's other public filings on EDGAR and SEDAR+, which may impact, among other things, the trading price and liquidity of the Company's common shares. . Forward-looking statements contained in this news release are expressly qualified by this cautionary statement and reflect our expectations as of the date hereof and thus are subject to change thereafter. The Company disclaims any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law. Readers are further cautioned not to place undue reliance on forward-looking statements as there can be no assurance that the plans, intentions or expectations upon which they are placed will occur. Such information, although considered reasonable by management at the time of preparation, may prove to be incorrect and actual results may differ materially from those anticipated.
SOURCE: Medicus Pharma Ltd
View the original press release on ACCESS Newswire
A.Clark--AT


