
-
 No pressure on India opener Abhishek after two ducks, says coach
No pressure on India opener Abhishek after two ducks, says coach
-
Sakamoto eyes figure skating gold in Olympic farewell

-
 Pereira 'trusts' Forest owner Marinakis despite three sackings this season
Pereira 'trusts' Forest owner Marinakis despite three sackings this season
-
AI 'arms race' risks human extinction, warns top computing expert

-
 Israeli bobsleigher dismisses Olympics 'diatribe' by Swiss TV commentator
Israeli bobsleigher dismisses Olympics 'diatribe' by Swiss TV commentator
-
Supreme leader says Iran can sink US warship as Geneva talks conclude

-
 Australia, Ireland out of T20 World Cup as Zimbabwe qualify after washout
Australia, Ireland out of T20 World Cup as Zimbabwe qualify after washout
-
Greece experts to examine Nazi atrocity photos find

-
 Los Angeles mayor calls for 2028 Olympics chairman to step down over Epstein files
Los Angeles mayor calls for 2028 Olympics chairman to step down over Epstein files
-
Evenepoel takes UAE Tour lead with time-trial win
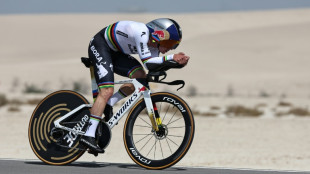
-
 Oil prices rise as Trump ramps up Iran threats
Oil prices rise as Trump ramps up Iran threats
-
EU investigates Shein over sale of childlike sex dolls

-
 Bangladesh's new PM, political heir Tarique Rahman
Bangladesh's new PM, political heir Tarique Rahman
-
Bangladesh's new PM Tarique Rahman takes power

-
 Rain threatens to knock Australia out of T20 World Cup
Rain threatens to knock Australia out of T20 World Cup
-
US civil rights leader Jesse Jackson dies at 84: family

-
 Trump's new envoy arrives in South Africa with relations frayed
Trump's new envoy arrives in South Africa with relations frayed
-
Jesse Jackson: civil rights lion sought 'common ground'

-
 Iran, United States hold new talks in Geneva
Iran, United States hold new talks in Geneva
-
At least 17 killed in two bomb attacks, gunfight in northwest Pakistan

-
 Tariq confident Pakistan can bounce back after India drubbing
Tariq confident Pakistan can bounce back after India drubbing
-
Being back in the USA 'feels amazing', says Vonn

-
 New Zealand cruise into Super Eights at T20 World Cup
New Zealand cruise into Super Eights at T20 World Cup
-
Moscow, Kyiv meet for US-brokered talks after fresh attacks

-
 Exhilarating Italy aim to sign off with giant-killing at T20 World Cup
Exhilarating Italy aim to sign off with giant-killing at T20 World Cup
-
Samra hits 110 for Canada against New Zealand at T20 World Cup

-
 'Made in Europe' or 'Made with Europe'? Buy European push splits bloc
'Made in Europe' or 'Made with Europe'? Buy European push splits bloc
-
Slovakia revamps bunkers with Ukraine war uncomfortably close

-
 Sydney man jailed for mailing reptiles in popcorn bags
Sydney man jailed for mailing reptiles in popcorn bags
-
'Like a Virgin' songwriter Billy Steinberg dies at 75

-
 Who fills Sexton vacuum? Irish fly-half debate no closer to resolution
Who fills Sexton vacuum? Irish fly-half debate no closer to resolution
-
Japan hails 'new chapter' with first Olympic pairs skating gold

-
 Russian prosthetics workshops fill up with wounded soldiers
Russian prosthetics workshops fill up with wounded soldiers
-
'Not just props that eat': Extras seek recognition at their own 'Oscars'

-
 Bangladesh PM-to-be Tarique Rahman and lawmakers sworn into parliament
Bangladesh PM-to-be Tarique Rahman and lawmakers sworn into parliament
-
At least 14 killed in spate of attacks in northwest Pakistan

-
 Peru Congress to debate impeachment of interim president
Peru Congress to debate impeachment of interim president
-
Bleak future for West Bank pupils as budget cuts bite

-
 Oil in spotlight as Trump's Iran warning rattles sleepy markets
Oil in spotlight as Trump's Iran warning rattles sleepy markets
-
Why are more under-50s getting colorectal cancer? 'We don't know'

-
 Moscow, Kyiv set for Geneva peace talks amid Russian attacks
Moscow, Kyiv set for Geneva peace talks amid Russian attacks
-
Iran, United States set for new talks in Geneva

-
 China has slashed air pollution, but the 'war' isn't over
China has slashed air pollution, but the 'war' isn't over
-
India's tougher AI social media rules spark censorship fears

-
 Doctors, tourism, tobacco: Cuba buckling under US pressure
Doctors, tourism, tobacco: Cuba buckling under US pressure
-
Indonesia capital faces 'filthy' trash crisis

-
 France grants safe haven to anti-Kremlin couple detained by ICE
France grants safe haven to anti-Kremlin couple detained by ICE
-
ClearSign Receives Fifth Low-Emission Flare Order from an Energy Company in California
-
 The Glimpse Group Reports Q2 Fiscal Year 2026 Financial Results
The Glimpse Group Reports Q2 Fiscal Year 2026 Financial Results
-
Stabilis Solutions Announces Preliminary Fourth Quarter 2025 Results, Provides Business Update


Moderna Receives European Commission Marketing Authorization for COVID-19 Vaccine mNEXSPIKE
mNEXSPIKE is the third Moderna vaccine authorized in the European Union, strengthening the Company's respiratory vaccine portfolio in Europe
CAMBRIDGE, MA / ACCESS Newswire / February 17, 2026 / Moderna, Inc. (NASDAQ:MRNA) today announced that the European Commission (EC) has granted marketing authorization for mNEXSPIKE® (mRNA-1283), a new COVID vaccine, for active immunization to prevent COVID-19 caused by SARS-CoV-2 in individuals aged 12 years and older.
This marketing authorization follows the positive opinion adopted by the European Medicines Agency's (EMA) Committee for Medicinal Products for Human Use (CHMP) and marks Moderna's third product authorized in the European Union, alongside Spikevax® and mRESVIA®, further strengthening the Company's respiratory vaccine portfolio in Europe. The marketing authorization is valid in all 27 European Union Member States, as well as Iceland, Liechtenstein and Norway. Moderna expects to make mNEXSPIKE available in Europe pending regulatory timelines and local market access pathways.
"We welcome the European Commission's decision, which reflects the strength of the scientific data supporting mNEXSPIKE and our continued commitment to advancing innovative vaccines for populations most at risk," said Stéphane Bancel, Chief Executive Officer of Moderna. "COVID-19 has become an endemic respiratory disease, with older adults continuing to bear a disproportionate burden of severe outcomes. Europe represents a key region for respiratory vaccines, and we are pleased to have a new vaccine available to help protect Europeans when the EU COVID market reopens starting this year in some markets."
The EC decision is supported by results from a randomized, observer-blind, active-controlled Phase 3 clinical trial (EudraCT: 2023-000884-30; ClinicalTrials.gov: NCT05815498), which enrolled approximately 11,400 participants aged 12 years and older. The primary efficacy objective of the study was to demonstrate non-inferior vaccine efficacy against COVID-19 starting 14 days after mNEXSPIKE compared to that after the comparator vaccine, mRNA-1273 (Spikevax), Moderna's original COVID-19 vaccine. In the trial, participants received either a 10 μg dose of mNEXSPIKE or a 50 μg dose of Spikevax. mNEXSPIKE showed a 9.3% higher relative vaccine efficacy compared to Spikevax in individuals aged 12 years and older, and in a descriptive subgroup analysis, a 13.5% higher relative vaccine efficacy in adults aged 65 years and older.
In the Phase 3 trial, mNEXSPIKE was found to have a similar safety profile to Spikevax, with fewer local reactions and comparable systemic reactions. The most commonly solicited adverse reactions were injection pain, fatigue, headache and myalgia.
Moderna has already received regulatory approval for mNEXSPIKE in the U.S., Canada and Australia, and continues to pursue approvals in additional markets worldwide.
About Moderna
Moderna is a pioneer and leader in the field of mRNA medicine. Through the advancement of its technology platform, Moderna is reimagining how medicines are made to transform how we treat and prevent diseases. Since its founding, Moderna's mRNA platform has enabled the development of vaccines and therapeutics across infectious diseases, cancer, rare diseases and more.
With a global team and a unique culture, driven by the company's values and mindsets, Moderna's mission is to deliver the greatest possible impact to people through mRNA medicines. For more information about Moderna, please visit modernatx.com and connect with us on X, Facebook, Instagram, YouTube and LinkedIn.
mNEXSPIKE®, Spikevax® and mRESVIA® are registered trademarks of Moderna.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including statements regarding: the availability of mNEXSPIKE in Europe; the opportunity in the European respiratory vaccine market and Moderna's geographical expansion; the safety profile of mNEXSPIKE; and potential approvals in additional markets worldwide. In some cases, forward-looking statements can be identified by terminology such as "will," "may," "should," "could," "expects," "intends," "plans," "aims," "anticipates," "believes," "estimates," "predicts," "potential," "continue," or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. The forward-looking statements in this press release are neither promises nor guarantees, and you should not place undue reliance on these forward-looking statements because they involve known and unknown risks, uncertainties, and other factors, many of which are beyond Moderna's control and which could cause actual results to differ materially from those expressed or implied by these forward-looking statements. These risks, uncertainties, and other factors include, among others, those risks and uncertainties described under the heading "Risk Factors" in Moderna's Annual Report on Form 10-K for the fiscal year ended December 31, 2024, filed with the U.S. Securities and Exchange Commission (SEC), and in subsequent filings made by Moderna with the SEC, which are available on the SEC's website at www.sec.gov. Except as required by law, Moderna disclaims any intention or responsibility for updating or revising any forward-looking statements contained in this press release in the event of new information, future developments or otherwise. These forward-looking statements are based on Moderna's current expectations and speak only as of the date of this press release.
Moderna Contacts
Media:
Chris Ridley
Head of Global Media Relations
+1 617-800-3651
[email protected]
Investors:
Lavina Talukdar
Senior Vice President & Head of Investor Relations
+1 617-209-5834
[email protected]
SOURCE: Moderna, Inc.
View the original press release on ACCESS Newswire
M.Robinson--AT

