
-
 Stocks fall as tech valuation fears stoke volatility
Stocks fall as tech valuation fears stoke volatility
-
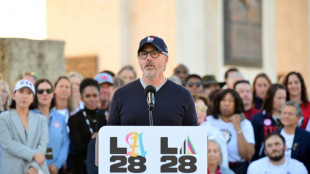
US Olympic body backs LA28 leadership amid Wasserman scandal
-
 Gnabry extends Bayern Munich deal until 2028
Gnabry extends Bayern Munich deal until 2028
-
England captain Stokes suffers facial injury after being hit by ball

-
 Italy captain Lamaro amongst trio set for 50th caps against Scotland
Italy captain Lamaro amongst trio set for 50th caps against Scotland
-
Piastri plays down McLaren rivalry with champion Norris

-
 ECB holds interest rates as strong euro causes jitters
ECB holds interest rates as strong euro causes jitters
-
Spain, Portugal face floods and chaos after deadly new storm

-
 EU close to sealing trade deal with Australia
EU close to sealing trade deal with Australia
-
German Cup final to stay in Berlin until 2030

-
 What does Iran want from talks with the US?
What does Iran want from talks with the US?
-
Taming the lion: Olympians take on Bormio's terrifying Stelvio piste

-
 Wind turbine maker Vestas sees record revenue in 2025
Wind turbine maker Vestas sees record revenue in 2025
-
Italy's Casse tops second Olympic downhill training

-
 Anti-doping boss 'uncomfortable' with Valieva's coach at Olympics
Anti-doping boss 'uncomfortable' with Valieva's coach at Olympics
-
Bitcoin under $70,000 for first time since Trump's election

-
 'I am sorry,' embattled UK PM tells Epstein victims
'I am sorry,' embattled UK PM tells Epstein victims
-
England's Brook predicts record 300-plus scores at T20 World Cup

-
 Ukraine, Russia swap prisoners, US says 'work remains' to end war
Ukraine, Russia swap prisoners, US says 'work remains' to end war
-
Wales' Rees-Zammit at full-back for Six Nations return against England

-
 Sad horses and Draco Malfoy: China's unexpected Lunar New Year trends
Sad horses and Draco Malfoy: China's unexpected Lunar New Year trends
-
Hong Kong students dissolve pro-democracy group under 'severe' pressure

-
 Germany claws back 59 mn euros from Amazon over price controls
Germany claws back 59 mn euros from Amazon over price controls
-
Germany claws back 70 mn euros from Amazon over price controls

-
 VW and Stellantis urge help to keep carmaking in Europe
VW and Stellantis urge help to keep carmaking in Europe
-
Stock markets drop amid tech concerns before rate calls

-
 BBVA posts record profit after failed Sabadell takeover
BBVA posts record profit after failed Sabadell takeover
-
UN human rights agency in 'survival mode': chief

-
 Greenpeace slams fossil fuel sponsors for Winter Olympics
Greenpeace slams fossil fuel sponsors for Winter Olympics
-
Greenpeace slams fossel fuel sponsors for Winter Olympics

-
 Kinghorn, Van der Merwe dropped by Scotland for Six Nations opener
Kinghorn, Van der Merwe dropped by Scotland for Six Nations opener
-
Russia says thwarted smuggling of giant meteorite to UK

-
 Salt war heats up in ice-glazed Berlin
Salt war heats up in ice-glazed Berlin
-
Liverpool in 'good place' for years to come, says Slot

-
 Heathrow still Europe's busiest airport, but Istanbul gaining fast
Heathrow still Europe's busiest airport, but Istanbul gaining fast
-
Highest storm alert lifted in Spain, one woman missing

-
 Shell profits climb despite falling oil prices
Shell profits climb despite falling oil prices
-
Pakistan will seek govt nod in potential India T20 finals clash

-
 China shuns calls to enter nuclear talks after US-Russia treaty lapses
China shuns calls to enter nuclear talks after US-Russia treaty lapses
-
German factory orders rise at fastest rate in 2 years in December

-
 Nigeria president deploys army after new massacre
Nigeria president deploys army after new massacre
-
Ukraine, Russia, US start second day of war talks

-
 Nepal's youth lead the charge in the upcoming election
Nepal's youth lead the charge in the upcoming election
-
Sony hikes forecasts even as PlayStation falters

-
 Rijksmuseum puts the spotlight on Roman poet's epic
Rijksmuseum puts the spotlight on Roman poet's epic
-
Trump fuels EU push to cut cord with US tech

-
 Fearless talent: Five young players to watch at the T20 World Cup
Fearless talent: Five young players to watch at the T20 World Cup
-
India favourites as T20 World Cup to begin after chaotic build-up

-
 Voter swings raise midterm alarm bells for Trump's Republicans
Voter swings raise midterm alarm bells for Trump's Republicans
-
Australia dodges call for arrest of visiting Israel president


Telomir Pharmaceuticals Reports New Data Supporting an Epigenetic Modulation Mechanism Implicated in Cancer and Aging
Cellular findings show Telomir-Zn modulates intracellular metal balance linked to oxidative stress, mitochondrial dysfunction, DNA methylation instability, and genomic integrity-without relying on cytotoxic mechanisms.
MIAMI, FLORIDA / ACCESS Newswire / February 5, 2026 / Telomir Pharmaceuticals, Inc. (NASDAQ:TELO) ("Telomir" or the "Company"), a preclinical-stage biotechnology company developing small-molecule therapeutics targeting fundamental biological mechanisms implicated in cancer, aging, and degenerative disease, today announced new cellular study results demonstrating that Telomir-1, in the form of Telomir-Zn, induces a rapid and coordinated intracellular redistribution of zinc and iron.
These findings extend Telomir's previously reported intracellular iron-reduction data by directly demonstrating, for the first time, that Telomir-Zn simultaneously increases intracellular zinc while reducing redox-active ferrous iron inside living cells. The coupled nature of these effects supports a differentiated intracellular metal-modulating mechanism rather than simple extracellular metal chelation.
Why This Biology Matters in Cancer and Aging
Cancer and accelerated aging are increasingly understood to share common upstream biological drivers, including dysregulated metal homeostasis, excess oxidative stress, mitochondrial dysfunction, epigenetic instability, and cumulative genomic damage.
Redox-active metals such as iron and copper can catalyze the formation of reactive oxygen species (ROS), which, over time, contribute to mitochondrial damage, oxidative DNA lesions, disruption of DNA methylation patterns, and telomere attrition. These processes are closely associated with epigenetic drift, impaired DNA repair, and genomic instability-hallmarks observed across both tumor biology and age-associated cellular decline.
Zinc plays a distinct biological role. Unlike iron and copper, zinc is redox-inert under physiological conditions and supports chromatin structure, DNA repair, antioxidant defense systems, and telomere-associated genomic stability. Maintaining appropriate intracellular zinc availability while limiting excess redox-active metals is therefore central to preserving cellular function over time.
Study Overview and Key Findings
To assess whether Telomir-Zn alters intracellular metal pools, Telomir Pharmaceuticals, in collaboration with Smart Assays Biotechnologies, has quantified labile intracellular zinc and iron levels in cultured human HaCaT cells using complementary live-cell fluorescent probes.
Key observations include:
Rapid, dose-dependent zinc accumulation: Telomir-Zn exposure resulted in a measurable increase in intracellular zinc within 30 minutes, sustained over a two-hour period at low-micromolar concentrations, without loss of cell confluence or viability.
Reciprocal reduction of redox-active iron: Increasing Telomir-Zn concentrations were associated with progressive depletion of the intracellular ferrous iron pool, most closely linked to oxidative stress.
Coordinated intracellular modulation: Zinc accumulation and iron reduction occurred over similar concentration ranges and timeframes, supporting a coordinated intracellular process rather than independent or nonspecific metal effects.
Mechanistic Interpretation: Linking Metals, Mitochondria, Epigenetics, and Telomeres
Excess intracellular iron and copper are known to impair mitochondrial respiration, amplify ROS generation, and disrupt metal-dependent enzymes that regulate chromatin structure and DNA methylation. Several histone demethylases and DNA repair enzymes require tightly regulated Fe²⁺ availability, and metal imbalance can destabilize epigenetic control systems and accelerate genomic stress.
The observed Telomir-Zn-associated increase in intracellular zinc, coupled with a reduction of labile iron, is consistent with a proposed intracellular mechanism by which modulation of metal availability may attenuate oxidative stress while supporting zinc-dependent regulatory functions. These pathways are closely linked to mitochondrial health, epigenetic regulation, telomere maintenance, and long-term genomic stability, supporting the potential relevance of this mechanism across both oncology and age-associated disease biology.
Importantly, the epigenetically associated effects observed in these studies do not rely on inducing cellular damage or cytotoxic stress, distinguishing this approach from many existing epigenetic strategies that act through DNA damage or broad transcriptional disruption.
Management Commentary
"These findings link our earlier epigenetic and mitochondrial observations to a clear upstream mechanism-intracellular metal imbalance," said Erez Aminov, CEO of Telomir Pharmaceuticals. "By simultaneously reducing redox-active iron while introducing protective zinc, Telomir-Zn appears to influence oxidative stress, DNA methylation, and genomic stability in a way that does not rely on cellular damage. We believe this approach has important implications for how cancer and age-related disease may be addressed at their biological roots."
"For decades, cancer and age-related diseases have largely been approached by targeting downstream consequences-uncontrolled growth, accumulated damage, or end-stage dysfunction," said Dr. Itzchak Angel, Chief Scientific Advisor at Telomir Pharmaceuticals. "What is emerging here is a different biological strategy: addressing upstream drivers such as oxidative stress, mitochondrial instability, and epigenetic drift that are shared across these conditions. By demonstrating for the first time that Telomir-Zn can simultaneously modulate intracellular zinc and iron-key regulators of DNA methylation, redox balance, and telomere-associated genomic stability-these findings support a framework that could fundamentally change how we think about intervening in cancer and aging biology, without relying on toxicity or cellular injury."
Ongoing Activities and Upcoming Scientific Presentations
Telomir Pharmaceuticals plans to present data related to Telomir-Zn and its mechanism of action at several upcoming scientific and industry meetings, including:
16th World Congress on Breast Cancer Research & Therapies, March 23-24, 2026 (Paris, France)
AACR Annual Meeting 2026, April 17-22, 2026 (San Diego, CA)
BIO International Convention, June 22-25, 2026 (San Diego, CA)
3rd International Conference on Women's Health and Breast Cancer, October 5-6, 2026 (Tokyo, Japan)
IND Preparation and Ongoing Research Activities
Telomir Pharmaceuticals is finalizing IND-enabling activities for Telomir-Zn, including assembly of the required data package to support regulatory submission. The Company currently plans to submit an Investigational New Drug (IND) application in the first quarter of 2026.
In parallel, Telomir continues to advance a portfolio of ongoing and completed preclinical research programs, including studies in triple-negative breast cancer (TNBC) models and longevity-focused models, evaluating the biological relevance of Telomir-Zn's intracellular metal-modulating and epigenetically associated mechanisms.
Based on data generated from completed studies, manuscript submissions to peer-reviewed journals have been initiated, while additional data continue to be generated from ongoing preclinical studies. These efforts also support planned and upcoming scientific conference presentations.
About Telomir Pharmaceuticals
Telomir Pharmaceuticals, Inc. (NASDAQ:TELO) is a preclinical-stage biotechnology company developing small-molecule therapeutics designed to target fundamental epigenetic and metabolic mechanisms implicated in cancer, aging, and degenerative disease. The Company's lead program, Telomir-1 (Telomir-Zn), has demonstrated activity in preclinical studies involving modulation of intracellular metal homeostasis, redox balance, epigenetically regulated gene expression, mitochondrial function, and genomic stability.
Cautionary Note Regarding Forward-Looking Statements
This press release, statements of Telomir's management or advisors related thereto, and the statements contained in the news story linked in this release contain "forward-looking statements," which are statements other than historical facts made pursuant to the safe harbor provisions of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. These risks and uncertainties include, but are not limited to, the potential use of the data from our studies, our ability to develop and commercialize Telomir-1 for specific indications, and the safety of Telomir-1.
Any forward-looking statements in this press release are based on Telomir's current expectations, estimates and projections only as of the date of this release. These and other risks concerning Telomir's programs and operations are described in additional detail in its Annual Report on Form 10-K for the fiscal year ended December 31, 2024, which are on file with the SEC and available at www.sec.gov. Telomir explicitly disclaims any obligation to update any forward-looking statements except to the extent required by law.
Contact Information
Krystina Quintana
Email: [email protected]
Phone: (786) 396-6723
SOURCE: Telomir Pharmaceuticals, Inc
View the original press release on ACCESS Newswire
A.Clark--AT



