
-
 US Justice Dept releasing new batch of Epstein files
US Justice Dept releasing new batch of Epstein files
-
South Africa and Israel expel envoys in deepening feud

-
 French eyewear maker in spotlight after presidential showing
French eyewear maker in spotlight after presidential showing
-
Olympic dream 'not over', Vonn says after crash

-
 Brazil's Lula discharged after cataract surgery
Brazil's Lula discharged after cataract surgery
-
US Senate races to limit shutdown fallout as Trump-backed deal stalls

-
 'He probably would've survived': Iran targeting hospitals in crackdown
'He probably would've survived': Iran targeting hospitals in crackdown
-
Djokovic stuns Sinner to set up Australian Open final with Alcaraz

-
 Mateta omitted from Palace squad to face Forest
Mateta omitted from Palace squad to face Forest
-
Gold, silver prices tumble as investors soothed by Trump's Fed pick

-
 Trump attorney general orders arrest of ex-CNN anchor covering protests
Trump attorney general orders arrest of ex-CNN anchor covering protests
-
Djokovic 'pushed to the limit' in stunning late-night Sinner upset

-
 Tunisia's famed blue-and-white village threatened after record rains
Tunisia's famed blue-and-white village threatened after record rains
-
Top EU official voices 'shock' at Minneapolis violence

-
 Kremlin says agreed to halt strikes on Kyiv until Sunday
Kremlin says agreed to halt strikes on Kyiv until Sunday
-
Carrick calls for calm after flying start to Man Utd reign

-
 Djokovic to meet Alcaraz in Melbourne final after five-set marathon
Djokovic to meet Alcaraz in Melbourne final after five-set marathon
-
Italian officials to testify in trial over deadly migrant shipwreck

-
 Iran says defence capabilities 'never' up for negotiation
Iran says defence capabilities 'never' up for negotiation
-
UN appeals for more support for flood-hit Mozambicans

-
 Lijnders urges Man City to pile pressure on Arsenal in title race
Lijnders urges Man City to pile pressure on Arsenal in title race
-
Fulham sign Man City winger Oscar Bobb

-
 Strasbourg's Argentine striker Panichelli sets sights on PSG, World Cup
Strasbourg's Argentine striker Panichelli sets sights on PSG, World Cup
-
Jesus 'made love': Colombian president irks Christians with steamy claim

-
 IAEA board meets over Ukraine nuclear safety concerns
IAEA board meets over Ukraine nuclear safety concerns
-
Eurozone growth beats 2025 forecasts despite Trump woes

-
 Israel to partially reopen Gaza's Rafah crossing on Sunday
Israel to partially reopen Gaza's Rafah crossing on Sunday
-
Dutch PM-elect Jetten says not yet time to talk to Putin

-
 Social media fuels surge in UK men seeking testosterone jabs
Social media fuels surge in UK men seeking testosterone jabs
-
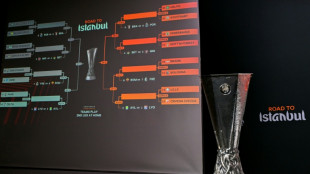
Forest face Fenerbahce, Celtic draw Stuttgart in Europa League play-offs
-
 US speed queen Vonn crashes at Crans-Montana, one week before Olympics
US speed queen Vonn crashes at Crans-Montana, one week before Olympics
-
Trump nominates former US Fed official as next central bank chief

-
 Alcaraz defends controversial timeout after beaten Zverev fumes
Alcaraz defends controversial timeout after beaten Zverev fumes
-
New Dutch government pledges ongoing Ukraine support

-
 Newcastle still coping with fallout from Isak exit, says Howe
Newcastle still coping with fallout from Isak exit, says Howe
-
Chad, France eye economic cooperation as they reset strained ties

-
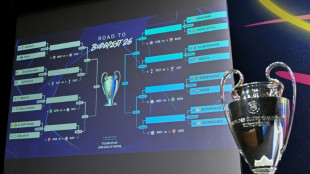 Real Madrid to play Benfica, PSG face Monaco in Champions League play-offs
Real Madrid to play Benfica, PSG face Monaco in Champions League play-offs
-
Everton winger Grealish set to miss rest of season in World Cup blow

-
 Trump brands Minneapolis nurse killed by federal agents an 'agitator'
Trump brands Minneapolis nurse killed by federal agents an 'agitator'
-
Arteta focuses on the positives despite Arsenal stumble

-
 Fijian Drua sign France international back Vakatawa
Fijian Drua sign France international back Vakatawa
-
Kevin Warsh, a former Fed 'hawk' now in tune with Trump

-
 Zverev rails at Alcaraz timeout in 'one of the best battles ever'
Zverev rails at Alcaraz timeout in 'one of the best battles ever'
-
Turkey leads Iran diplomatic push as Trump softens strike threat

-
 Zelensky backs energy ceasefire, Russia bombs Ukraine despite Trump intervention
Zelensky backs energy ceasefire, Russia bombs Ukraine despite Trump intervention
-
'Superman' Li Ka-shing, Hong Kong billionaire behind Panama ports deal

-
 Skiing great Lindsey Vonn crashes at Crans-Montana, one week before Olympics
Skiing great Lindsey Vonn crashes at Crans-Montana, one week before Olympics
-
Slot warns Liverpool 'can't afford mistakes' in top-four scrap

-
 Paris show by late Martin Parr views his photos through political lens
Paris show by late Martin Parr views his photos through political lens
-
'Believing' Alcaraz outlasts Zverev in epic to reach maiden Melbourne final

| RBGPF | 1.65% | 83.78 | $ | |
| SCS | 0.12% | 16.14 | $ | |
| RYCEF | -2.69% | 16 | $ | |
| NGG | -0.33% | 84.77 | $ | |
| CMSC | -0.02% | 23.69 | $ | |
| AZN | 0.38% | 92.94 | $ | |
| GSK | 1.03% | 51.18 | $ | |
| BP | 0.09% | 38.075 | $ | |
| BTI | -0.47% | 59.93 | $ | |
| RIO | -3.08% | 92.29 | $ | |
| VOD | -0.55% | 14.63 | $ | |
| BCE | 0.1% | 25.51 | $ | |
| JRI | 0.23% | 12.985 | $ | |
| BCC | -1.31% | 79.132 | $ | |
| RELX | -1.25% | 35.72 | $ | |
| CMSD | 0.05% | 24.073 | $ |

Ensysce Biosciences Provides Enrollment Update on Pivotal Phase 3 Trial of PF614, Its Next-Generation Opioid for Severe Acute Pain
~ Engineered to Deliver Potent Pain Relief with Built-In Abuse Protection ~
SAN DIEGO, CA / ACCESS Newswire / January 28, 2026 / Ensysce Biosciences, Inc. (NASDAQ:ENSC) ("Ensysce" or the "Company"), a clinical-stage pharmaceutical company pioneering novel solutions for severe pain with built-in abuse and overdose protection, today announced it has enrolled 50% of subjects targeted for interim review in its pivotal Phase 3 clinical trial of PF614, the Company's next-generation opioid candidate engineered to deliver powerful pain relief with built-in abuse protection..
Enrollment began in late December 2025 and is progressing rapidly across three U.S. clinical sites: CenExel JBR (Salt Lake City, Utah); CenExel Atlanta (Decatur, Georgia); and ERG-HD Research, LLC (Houston, Texas). The study is being led by Dr. Todd Bertoch, Dr. Jessica McCoun, and Dr. D'Aunno, recognized experts in anesthesiology and pain management.
The pivotal PF614-301 trial is a multicenter, randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of PF614 for the treatment of moderate to severe pain following abdominoplasty. The study is designed to demonstrate PF614's ability to deliver consistent, clinically meaningful post-surgical pain relief using twice-daily dosing.
PF614 leverages Ensysce's proprietary chemical activation technology, which is designed to keep the opioid inactive until swallowed, limiting the impact of tampering and dose manipulation while enabling extended-release pain control. This approach is intended to address one of the central challenges in modern pain care: delivering opioid-level efficacy while reducing the risks of abuse and misuse.
"This milestone reflects the strength of our execution to provide better options to treat severe acute pain," said Dr. Lynn Kirkpatrick, Chief Executive Officer of Ensysce. "Patients recovering from major surgery still require opioid level analgesia for effective pain control. PF614 is designed to deliver that level of relief reliably and predictably while incorporating intrinsic safeguards that are absent from conventional opioids. Achieving this enrollment milestone in the early stages of this Phase 3 program brings us meaningfully closer to delivering interim data and advancing what we believe could be a new standard in acute pain management. We are pleased to share this encouraging study update."
About Ensysce Biosciences
Ensysce Biosciences is a clinical-stage pharmaceutical company disrupting the pain treatment landscape with its proprietary Trypsin-Activated Abuse Protection (TAAP™) and Multi-Pill Abuse Resistance (MPAR®) platforms. By engineering opioids with intrinsic safeguards against tampering, misuse, and overdose, Ensysce aims to offer safer, life-saving options for patients in need of powerful pain relief. Learn more at: www.ensysce.com
Forward-Looking Statements
Statements contained in this press release that are not purely historical may be deemed to be forward-looking statements for the purposes of the safe harbor provisions under The Private Securities Litigation Reform Act of 1995 and other federal securities laws. Without limiting the foregoing, the use of words such as "may," "intends," "can," "might," "will," "expect," "plan," "possible," "believe" and other similar expressions are intended to identify forward-looking statements. The product candidates discussed are in clinic and not approved and there can be no assurance that the clinical programs will be successful in demonstrating safety and/or efficacy, that Ensysce will not encounter problems or delays in clinical development, or that any product candidate will ever receive regulatory approval or be successfully commercialized. All forward-looking statements are based on estimates and assumptions by Ensysce's management that, although Ensysce believes to be reasonable, are inherently uncertain. All forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those that Ensysce expected. In addition, Ensysce's business is subject to additional risks and uncertainties, including among others, the initiation and conduct of preclinical studies and clinical trials; the timing and availability of data from preclinical studies and clinical trials; expectations for regulatory submissions and approvals; potential safety concerns related to, or efficacy of, Ensysce's product candidates; the availability or commercial potential of product candidates; the ability of Ensysce to fund its continued operations, including its planned clinical trials; the dilutive effect of stock issuances from our fundraising; and Ensysce's and its partners' ability to perform under their license, collaboration and manufacturing arrangements. These statements are also subject to a number of material risks and uncertainties that are described in Ensysce's most recent quarterly report on Form 10-Q and current reports on Form 8-K, which are available, free of charge, at the SEC's website at www.sec.gov. Any forward-looking statement speaks only as of the date on which it was made. Ensysce undertakes no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise, except as required under applicable law.
Ensysce Biosciences Company Contact:
Lynn Kirkpatrick, Ph.D.
Chief Executive Officer
(858) 263-4196
Ensysce Biosciences Investor Relations Contact:
Shannon Devine
MZ North America
Main: 203-741-8811
[email protected]
SOURCE: Ensysce Biosciences
View the original press release on ACCESS Newswire
M.King--AT
