
-
 Trump orders marijuana reclassified as less dangerous drug
Trump orders marijuana reclassified as less dangerous drug
-
Rams ace Nacua apologizes over 'antisemitic' gesture furor

-
 McIlroy wins BBC sports personality award for 2025 heroics
McIlroy wins BBC sports personality award for 2025 heroics
-
Napoli beat Milan in Italian Super Cup semi-final

-
 Violence erupts in Bangladesh after wounded youth leader dies
Violence erupts in Bangladesh after wounded youth leader dies
-
EU-Mercosur deal delayed as farmers stage Brussels show of force

-
 US hosting new Gaza talks to push next phase of deal
US hosting new Gaza talks to push next phase of deal
-
Chicago Bears mulling Indiana home over public funding standoff

-
 Trump renames Kennedy arts center after himself
Trump renames Kennedy arts center after himself
-
Trump rebrands housing supplement as $1,776 bonuses for US troops

-
 Harrison Ford to get lifetime acting award
Harrison Ford to get lifetime acting award
-
Trump health chief seeks to bar trans youth from gender-affirming care

-
 Argentine unions in the street over Milei labor reforms
Argentine unions in the street over Milei labor reforms
-
Trump signs order reclassifying marijuana as less dangerous

-
 Famed Kennedy arts center to be renamed 'Trump-Kennedy Center'
Famed Kennedy arts center to be renamed 'Trump-Kennedy Center'
-
US accuses S.Africa of harassing US officials working with Afrikaners

-
 Brazil open to EU-Mercosur deal delay as farmers protest in Brussels
Brazil open to EU-Mercosur deal delay as farmers protest in Brussels
-
Wounded Bangladesh youth leader dies in Singapore hospital

-
 New photo dump fuels Capitol Hill push on Epstein files release
New photo dump fuels Capitol Hill push on Epstein files release
-
Brazil, Mexico seek to defuse US-Venezuela crisis

-
 Assange files complaint against Nobel Foundation over Machado win
Assange files complaint against Nobel Foundation over Machado win
-
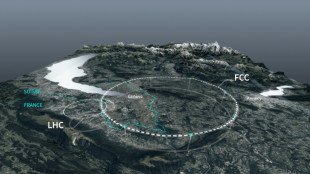
Private donors pledge $1 bn for CERN particle accelerator
-
 Russian court orders Austrian bank Raiffeisen to pay compensation
Russian court orders Austrian bank Raiffeisen to pay compensation
-
US, Qatar, Turkey, Egypt to hold Gaza talks in Miami

-
 Lula open to mediate between US, Venezuela to 'avoid armed conflict'
Lula open to mediate between US, Venezuela to 'avoid armed conflict'
-
Brussels farmer protest turns ugly as EU-Mercosur deal teeters

-
 US imposes sanctions on two more ICC judges for Israel probe
US imposes sanctions on two more ICC judges for Israel probe
-
US accuses S. Africa of harassing US officials working with Afrikaners

-
 ECB holds rates as Lagarde stresses heightened uncertainty
ECB holds rates as Lagarde stresses heightened uncertainty
-
Trump Media announces merger with fusion power company

-
 Stocks rise as US inflation cools, tech stocks bounce
Stocks rise as US inflation cools, tech stocks bounce
-
Zelensky presses EU to tap Russian assets at crunch summit

-
 Pope replaces New York's Cardinal Dolan with pro-migrant bishop
Pope replaces New York's Cardinal Dolan with pro-migrant bishop
-
Odermatt takes foggy downhill for 50th World Cup win

-
 France exonerates women convicted over abortions before legalisation
France exonerates women convicted over abortions before legalisation
-
UK teachers to tackle misogyny in classroom

-
 Historic Afghan cinema torn down for a mall
Historic Afghan cinema torn down for a mall
-
US consumer inflation cools unexpectedly in November

-
 Danish 'ghetto' residents upbeat after EU court ruling
Danish 'ghetto' residents upbeat after EU court ruling
-
ECB holds rates but debate swirls over future

-
 Pope replaces New York's Cardinal Timothy Dolan with little-known bishop
Pope replaces New York's Cardinal Timothy Dolan with little-known bishop
-
Bank of England cuts interest rate after UK inflation slides

-
 Have Iran's authorities given up on the mandatory hijab?
Have Iran's authorities given up on the mandatory hijab?
-
Spain to buy 100 military helicopters from Airbus

-
 US strike on alleged drug boat in Pacific kills four
US strike on alleged drug boat in Pacific kills four
-
Thailand strikes building in Cambodia's border casino hub

-
 Protests in Bangladesh as India cites security concerns
Protests in Bangladesh as India cites security concerns
-
European stocks rise before central bank decisions on rates

-
 Tractors clog Brussels in anger at EU-Mercosur trade deal
Tractors clog Brussels in anger at EU-Mercosur trade deal
-
Not enough evidence against Swedish PM murder suspect: prosecutor


CEPI to Fund Pivotal Phase 3 Trial for Moderna's mRNA Pandemic Influenza Vaccine Candidate
Up to $54.3 million CEPI investment aims to help advance Moderna's H5 pandemic influenza vaccine candidate to licensure
Partnership strengthens global preparedness against a significant pandemic threat
If licensed and in the event of an influenza pandemic, Moderna will allocate 20% of its H5 pandemic vaccine manufacturing capacity for timely supply to low- and middle-income countries at affordable pricing
OSLO, NORWAY and CAMBRIDGE, MA / ACCESS Newswire / December 18, 2025 / The Coalition for Epidemic Preparedness Innovations (CEPI) will invest up to $54.3 million to support a pivotal Phase 3 clinical trial that aims to help advance Moderna's investigational mRNA-based H5 pandemic influenza vaccine candidate, mRNA-1018, to licensure. The funding marks a significant step forward in global pandemic preparedness that could enable fast, equitable access to vaccines for one of the world's most pressing health threats.
This Phase 3 study would be the first mRNA-based vaccine targeting pandemic influenza to enter a pivotal trial. If the vaccine candidate is licensed, it would expand the current global portfolio of H5 vaccines with a rapid-response platform that could revolutionize future pandemic responses, making a significant contribution to CEPI's 100 Days Mission, a global goal to develop safe and effective vaccines within 100 days of a new pandemic threat being identified.
Dr Richard Hatchett, Chief Executive Officer of CEPI said:
"Pandemic influenza remains one of the greatest threats to global health security. With this partnership, we are not just advancing vaccine science, we are fundamentally changing the game. By harnessing the speed and adaptability of mRNA technology, we could shave months off the response time, deliver vaccines at scale, and enable equitable access for all. This is how we plan to protect the world from the next flu pandemic."
Stéphane Bancel, Chief Executive Officer of Moderna said:
"We are proud to have the support of CEPI to advance our pandemic influenza vaccine candidate, research that is critical to our commitment to pandemic preparedness. mRNA technology can play a vital role in addressing emerging health threats quickly and effectively, and we look forward to continuing our partnership with CEPI as we advance our health security portfolio, and in parallel, further the 100 Days Mission."
A potential first-in-class mRNA vaccine for pandemic influenza
Conventional influenza vaccines require virus growth in eggs or cell culture, a process that can take months. By contrast, an mRNA vaccine can be designed in hours or days as soon as the virus's genetic sequence is known and swiftly manufactured at scale. The combination of speed, adaptability and scalability offered by mRNA technology is a potential critical advantage when a new pandemic strain emerges and every day that passes could cost lives.
If licensure is granted, Moderna is committed to working to provide people around the world with rapid, equitable access to the resulting H5 vaccine in the event of a pandemic. As part of this agreement, Moderna will allocate 20% of its H5 pandemic vaccine manufacturing capacity for timely supply to low- and middle-income countries at affordable pricing.
The Phase 3 trial, set to begin early in 2026, will evaluate the safety and immunogenicity of Moderna's H5 vaccine candidate in populations in the UK and U.S. It will build upon positive Phase 1/2 results which showed rapid and persistent immune responses in healthy adults aged 18 years and older. Potential licensure of the vaccine will also leverage data from a pivotal Phase 3 trial of Moderna's investigational seasonal influenza vaccine, mRNA-1010.
This project is part of CEPI and Moderna's strategic partnership, which aims to harness Moderna's mRNA platform to accelerate epidemic and pandemic vaccine development.
ENDS
About CEPI
CEPI is an innovative partnership between public, private, philanthropic, and civil organisations. Its mission is to accelerate the development of vaccines and other biologic countermeasures against epidemic and pandemic threats so they can be accessible to all people in need. CEPI has supported the development of more than 70 vaccine candidates or platform technologies against multiple known high-risk pathogens or a future Disease X. Central to CEPI's pandemic-beating plan is the '100 Days Mission' to accelerate the time taken to develop safe, effective and accessible vaccines against new threats to just 100 days. Learn more at CEPI.net.
About Moderna
Moderna is a pioneer and leader in the field of mRNA medicine. Through the advancement of its technology platform, Moderna is reimagining how medicines are made to transform how we treat and prevent diseases. Since its founding, Moderna's mRNA platform has enabled the development of vaccines and therapeutics across infectious diseases, cancer, rare diseases and more.
With a global team and a unique culture, driven by the company's values and mindsets, Moderna's mission is to deliver the greatest possible impact to people through mRNA medicines. For more information about Moderna, please visit modernatx.com and connect with us on X, Facebook, Instagram, YouTube and LinkedIn.
Moderna Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including statements regarding: CEPI's investment to advance Moderna's H5 pandemic influenza vaccine candidate; the potential for licensure of mRNA-1018; the safety and immunogenicity of mRNA-1018; the potential for mRNA technology to effectively address emerging health threats; and Moderna's ability to manufacture at scale. The forward-looking statements in this press release are neither promises nor guarantees, and you should not place undue reliance on these forward-looking statements because they involve known and unknown risks, uncertainties, and other factors, many of which are beyond Moderna's control and which could cause actual results to differ materially from those expressed or implied by these forward-looking statements. These risks, uncertainties, and other factors include, among others, those risks and uncertainties described under the heading "Risk Factors" in Moderna's Annual Report on Form 10-K for the fiscal year ended December 31, 2024, and in subsequent filings made by Moderna with the U.S. Securities and Exchange Commission, which are available on the SEC's website at www.sec.gov. Except as required by law, Moderna disclaims any intention or responsibility for updating or revising any forward-looking statements contained in this press release in the event of new information, future developments or otherwise. These forward-looking statements are based on Moderna's current expectations and speak only as of the date of this press release.
CEPI Media Contacts
Email: [email protected]
Phone: +44 7387 055214
Moderna Contacts
Media:
Chris Ridley
Head of Global Media Relations
+1 617-800-3651
[email protected]
Investors:
Lavina Talukdar
Senior Vice President & Head of Investor Relations
+1 617-209-5834
[email protected]
SOURCE: Moderna, Inc.
View the original press release on ACCESS Newswire
A.Taylor--AT

